| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 216880 | 1426310 | 2008 | 7 صفحه PDF | دانلود رایگان |
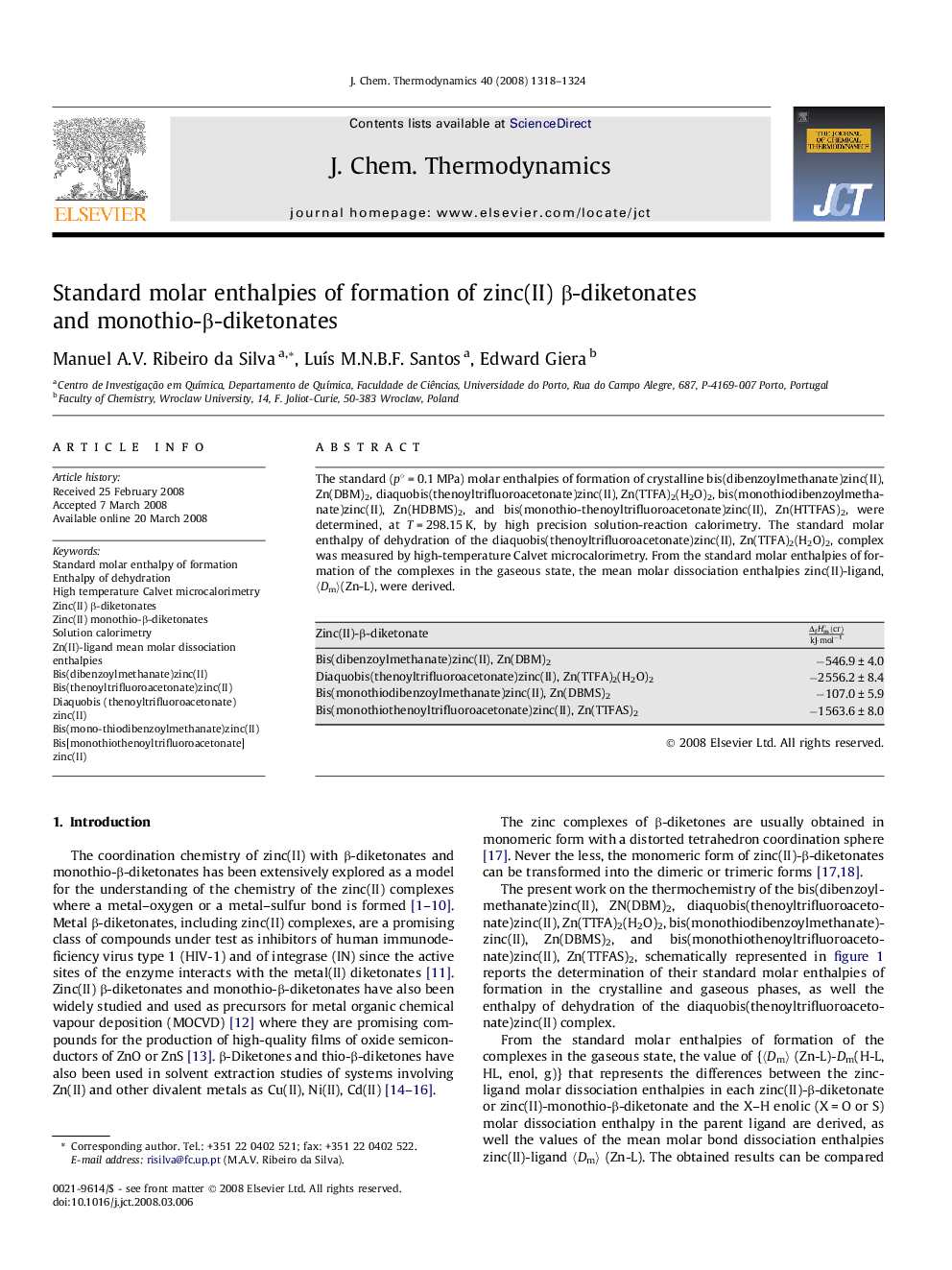
The standard (p∘ = 0.1 MPa) molar enthalpies of formation of crystalline bis(dibenzoylmethanate)zinc(II), Zn(DBM)2, diaquobis(thenoyltrifluoroacetonate)zinc(II), Zn(TTFA)2(H2O)2, bis(monothiodibenzoylmethanate)zinc(II), Zn(HDBMS)2, and bis(monothio-thenoyltrifluoroacetonate)zinc(II), Zn(HTTFAS)2, were determined, at T = 298.15 K, by high precision solution-reaction calorimetry. The standard molar enthalpy of dehydration of the diaquobis(thenoyltrifluoroacetonate)zinc(II), Zn(TTFA)2(H2O)2, complex was measured by high-temperature Calvet microcalorimetry. From the standard molar enthalpies of formation of the complexes in the gaseous state, the mean molar dissociation enthalpies zinc(II)-ligand, 〈Dm〉(Zn-L), were derived.Zinc(II)-β-diketonateΔfHm∘(cr)kJ·mol-1Bis(dibenzoylmethanate)zinc(II), Zn(DBM)2−546.9 ± 4.0Diaquobis(thenoyltrifluoroacetonate)zinc(II), Zn(TTFA)2(H2O)2−2556.2 ± 8.4Bis(monothiodibenzoylmethanate)zinc(II), Zn(DBMS)2−107.0 ± 5.9Bis(monothiothenoyltrifluoroacetonate)zinc(II), Zn(TTFAS)2−1563.6 ± 8.0Full-size tableTable optionsView in workspaceDownload as CSV
Journal: The Journal of Chemical Thermodynamics - Volume 40, Issue 8, August 2008, Pages 1318–1324