| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 229963 | 1427361 | 2016 | 10 صفحه PDF | دانلود رایگان |
• Microparticles and coprecipitates of ellagic acid have been obtained by SAS process.
• The negligible and stronger effects on particle size were identified.
• Concentration and temperature are two of the key factor to get a powder precipitation.
• The crystallinity of ellagic acid was unaltered after SAS process.
• Coprecipitates had faster ellagic acid release than microparticles in simulated fluids.
Microparticles of ellagic acid were prepared by a supercritical antisolvent process. The main parameters that influence this process were identified in order to carry out a subsequent coprecipitation using eudragit as a coating agent. The initial concentration of the solution and temperature play a significant role in the powder precipitation of ellagic acid; powders did not precipitate at concentrations below 20 mg/mL and temperatures above 40 °C. Higher CO2 and liquid solution flow rates together with smaller nozzle diameters are recommended in order to obtain smaller particles of ellagic acid. Taking into account these results, coprecipitates of ellagic acid and eudragit in different ratios were obtained under the best operating conditions. The coprecipitates were formed as spherical microparticles of eudragit surrounding sticks and flowers of ellagic acid. Dissolution profiles for commercial ellagic acid and processed microparticles were obtained in simulated fluids together with the release profiles of the coprecipitates. Commercial ellagic acid had a poor dissolution profile in simulated fluids. In general, ellagic acid was dissolved or released to a greater extent in simulated gastric fluids than in simulated intestinal fluids. Coprecipitates had faster release than microparticles of ellagic acid alone. The crystallinity and stability of SAS-processed ellagic acid remained unchanged.
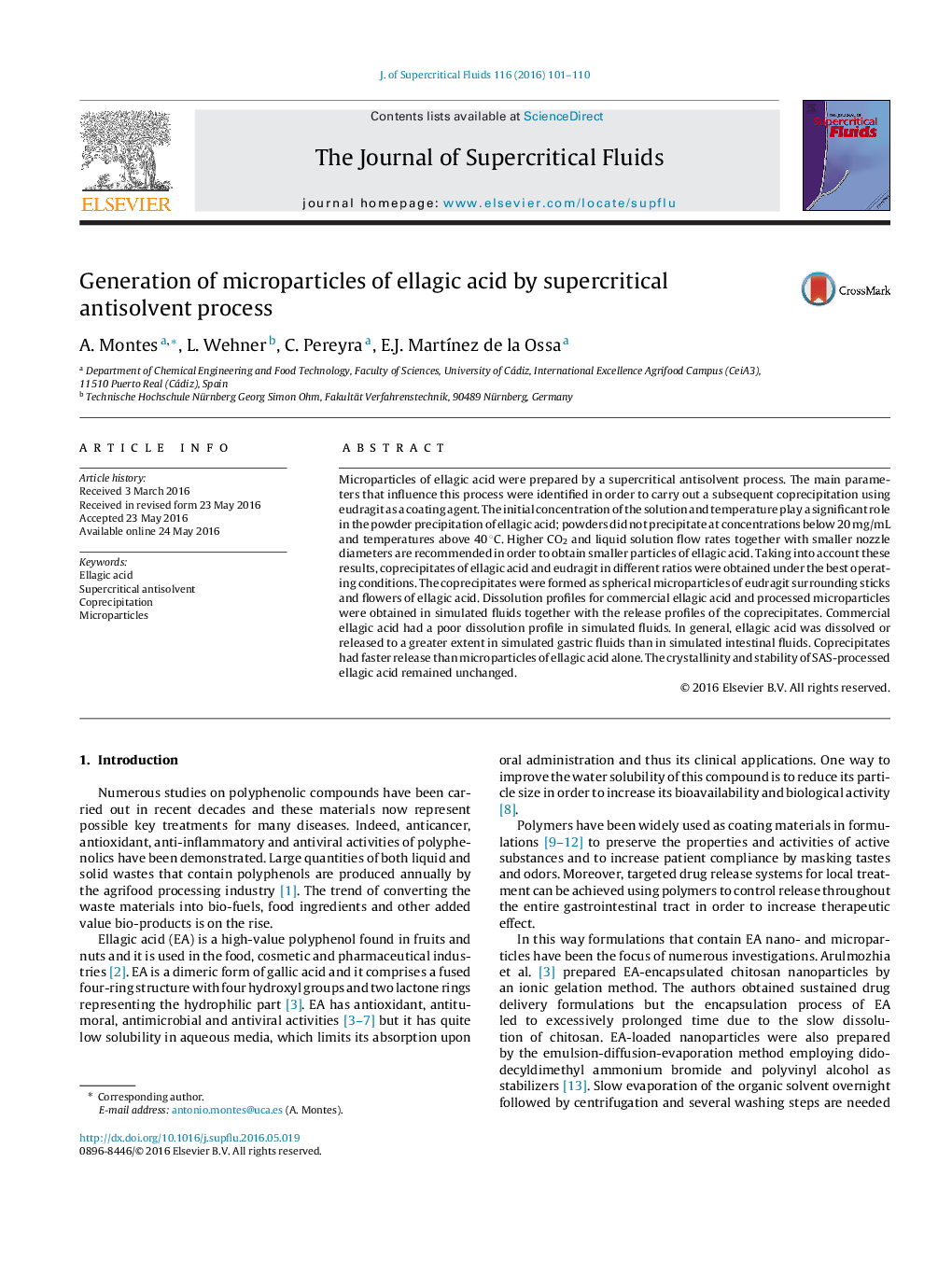
Figure optionsDownload as PowerPoint slideSEM images of SAS processed (a) ellagic acid, (b) eudragit and (c) coprecipitates ellagic acid-eudragit.
Journal: The Journal of Supercritical Fluids - Volume 116, October 2016, Pages 101–110