| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4370957 | 1617000 | 2016 | 7 صفحه PDF | دانلود رایگان |
• Trypanosoma cruzi's natural PPDK elutes as a homodimer and monomer.
• T. cruzi's PPDK homodimer is the more active form.
• Recombinant and natural PPDK show long delays (lag) in the activity progress curve.
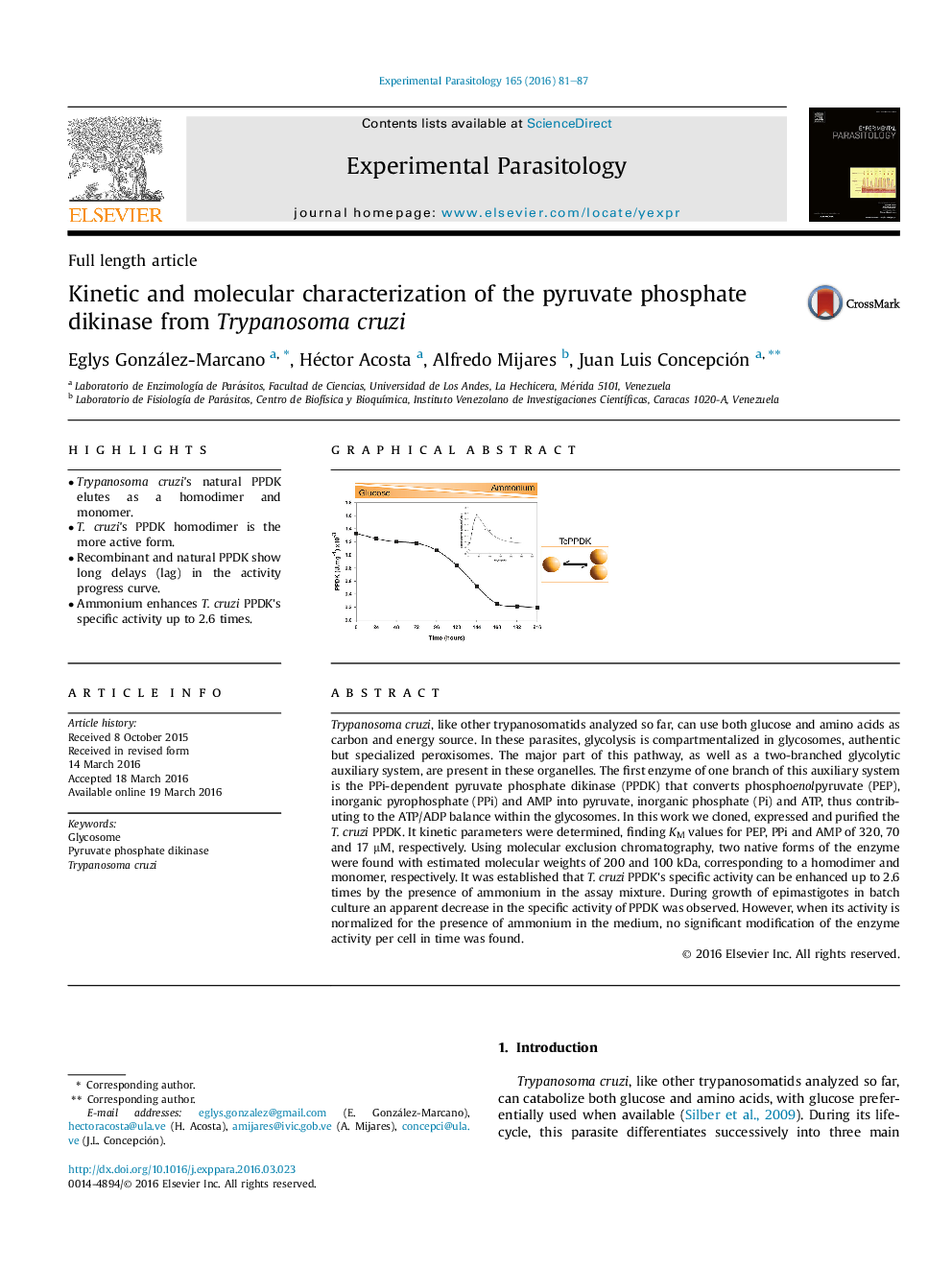
• Ammonium enhances T. cruzi PPDK's specific activity up to 2.6 times.
Trypanosoma cruzi, like other trypanosomatids analyzed so far, can use both glucose and amino acids as carbon and energy source. In these parasites, glycolysis is compartmentalized in glycosomes, authentic but specialized peroxisomes. The major part of this pathway, as well as a two-branched glycolytic auxiliary system, are present in these organelles. The first enzyme of one branch of this auxiliary system is the PPi-dependent pyruvate phosphate dikinase (PPDK) that converts phosphoenolpyruvate (PEP), inorganic pyrophosphate (PPi) and AMP into pyruvate, inorganic phosphate (Pi) and ATP, thus contributing to the ATP/ADP balance within the glycosomes. In this work we cloned, expressed and purified the T. cruzi PPDK. It kinetic parameters were determined, finding KM values for PEP, PPi and AMP of 320, 70 and 17 μM, respectively. Using molecular exclusion chromatography, two native forms of the enzyme were found with estimated molecular weights of 200 and 100 kDa, corresponding to a homodimer and monomer, respectively. It was established that T. cruzi PPDK's specific activity can be enhanced up to 2.6 times by the presence of ammonium in the assay mixture. During growth of epimastigotes in batch culture an apparent decrease in the specific activity of PPDK was observed. However, when its activity is normalized for the presence of ammonium in the medium, no significant modification of the enzyme activity per cell in time was found.
Figure optionsDownload as PowerPoint slide
Journal: Experimental Parasitology - Volume 165, June 2016, Pages 81–87