| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4371077 | 1617021 | 2014 | 9 صفحه PDF | دانلود رایگان |
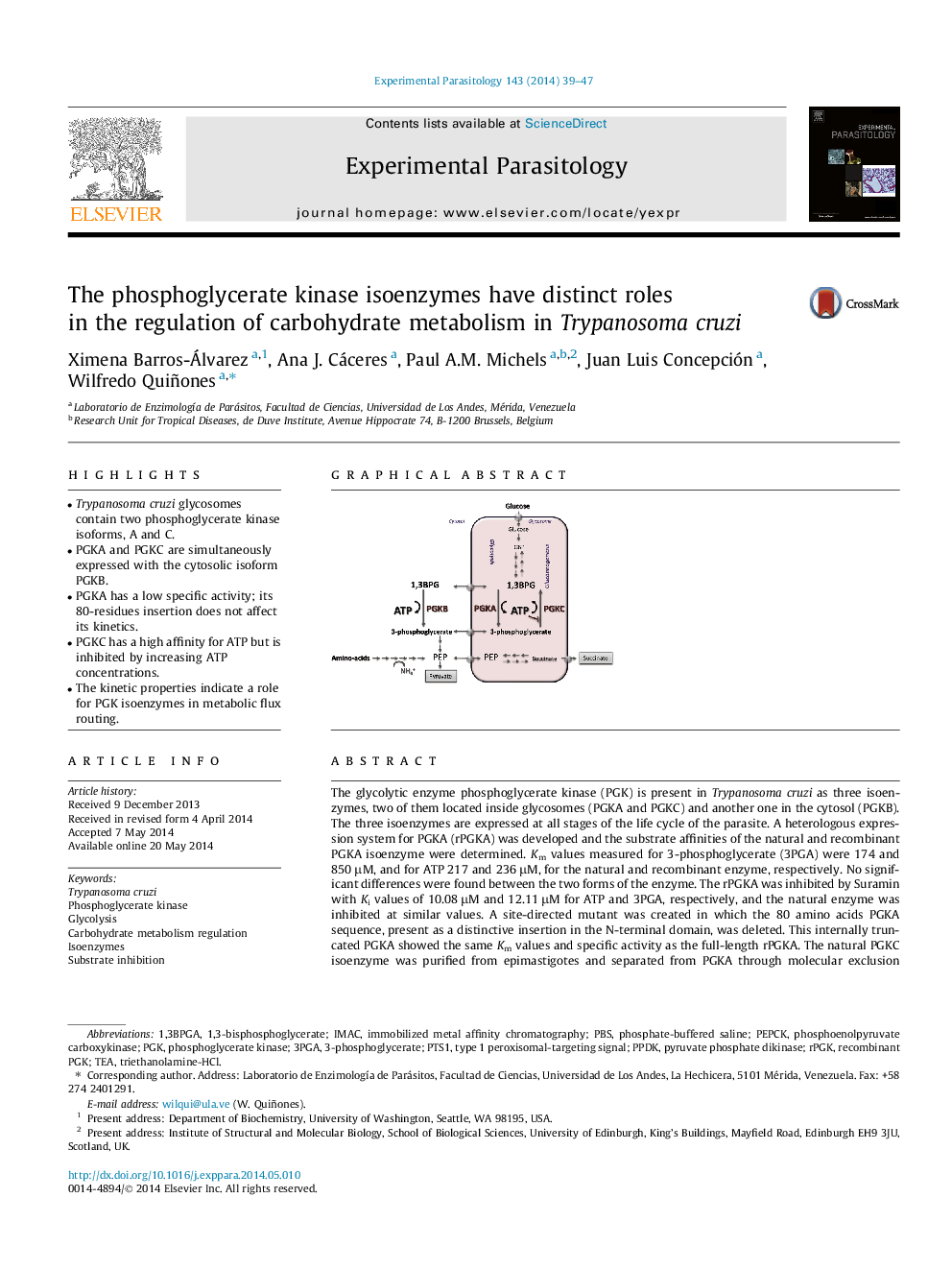
• Trypanosoma cruzi glycosomes contain two phosphoglycerate kinase isoforms, A and C.
• PGKA and PGKC are simultaneously expressed with the cytosolic isoform PGKB.
• PGKA has a low specific activity; its 80-residues insertion does not affect its kinetics.
• PGKC has a high affinity for ATP but is inhibited by increasing ATP concentrations.
• The kinetic properties indicate a role for PGK isoenzymes in metabolic flux routing.
The glycolytic enzyme phosphoglycerate kinase (PGK) is present in Trypanosoma cruzi as three isoenzymes, two of them located inside glycosomes (PGKA and PGKC) and another one in the cytosol (PGKB). The three isoenzymes are expressed at all stages of the life cycle of the parasite. A heterologous expression system for PGKA (rPGKA) was developed and the substrate affinities of the natural and recombinant PGKA isoenzyme were determined. Km values measured for 3-phosphoglycerate (3PGA) were 174 and 850 μM, and for ATP 217 and 236 μM, for the natural and recombinant enzyme, respectively. No significant differences were found between the two forms of the enzyme. The rPGKA was inhibited by Suramin with Ki values of 10.08 μM and 12.11 μM for ATP and 3PGA, respectively, and the natural enzyme was inhibited at similar values. A site-directed mutant was created in which the 80 amino acids PGKA sequence, present as a distinctive insertion in the N-terminal domain, was deleted. This internally truncated PGKA showed the same Km values and specific activity as the full-length rPGKA. The natural PGKC isoenzyme was purified from epimastigotes and separated from PGKA through molecular exclusion chromatography and its kinetic characteristics were determined. The Km value obtained for 3PGA was 192 μM, and 10 μM for ATP. Contrary to PGKA, the activity of PGKC is tightly regulated by ATP (substrate inhibition) with a Ki of 270 μM, suggesting a role for this isoenzyme in regulating metabolic fluxes inside the glycosomes.
Figure optionsDownload as PowerPoint slide
Journal: Experimental Parasitology - Volume 143, August 2014, Pages 39–47