| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5216344 | 1383260 | 2014 | 10 صفحه PDF | دانلود رایگان |
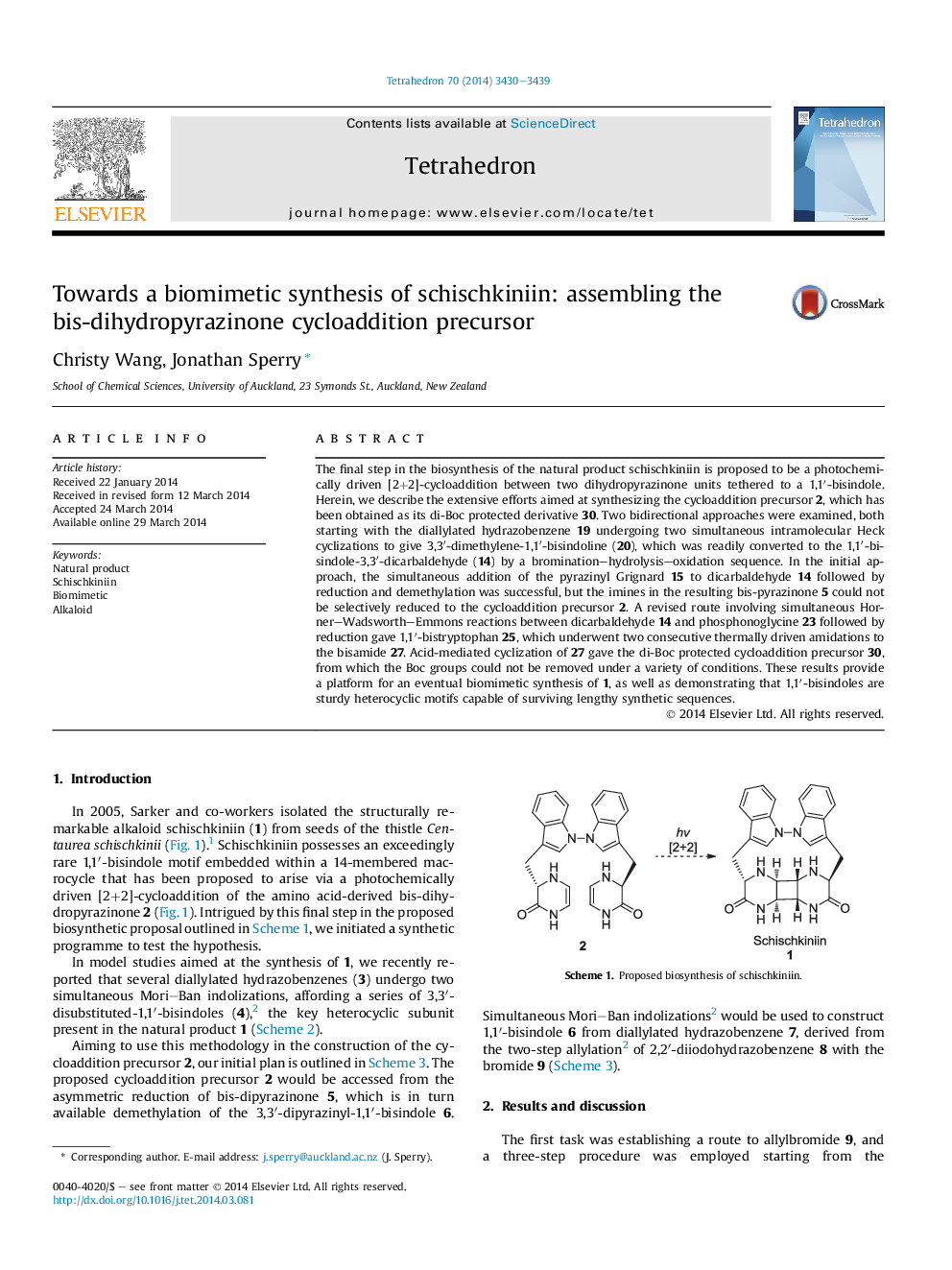
The final step in the biosynthesis of the natural product schischkiniin is proposed to be a photochemically driven [2+2]-cycloaddition between two dihydropyrazinone units tethered to a 1,1â²-bisindole. Herein, we describe the extensive efforts aimed at synthesizing the cycloaddition precursor 2, which has been obtained as its di-Boc protected derivative 30. Two bidirectional approaches were examined, both starting with the diallylated hydrazobenzene 19 undergoing two simultaneous intramolecular Heck cyclizations to give 3,3â²-dimethylene-1,1â²-bisindoline (20), which was readily converted to the 1,1â²-bisindole-3,3â²-dicarbaldehyde (14) by a bromination-hydrolysis-oxidation sequence. In the initial approach, the simultaneous addition of the pyrazinyl Grignard 15 to dicarbaldehyde 14 followed by reduction and demethylation was successful, but the imines in the resulting bis-pyrazinone 5 could not be selectively reduced to the cycloaddition precursor 2. A revised route involving simultaneous Horner-Wadsworth-Emmons reactions between dicarbaldehyde 14 and phosphonoglycine 23 followed by reduction gave 1,1â²-bistryptophan 25, which underwent two consecutive thermally driven amidations to the bisamide 27. Acid-mediated cyclization of 27 gave the di-Boc protected cycloaddition precursor 30, from which the Boc groups could not be removed under a variety of conditions. These results provide a platform for an eventual biomimetic synthesis of 1, as well as demonstrating that 1,1â²-bisindoles are sturdy heterocyclic motifs capable of surviving lengthy synthetic sequences.
Journal: Tetrahedron - Volume 70, Issue 21, 27 May 2014, Pages 3430-3439