| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5218202 | 1383319 | 2013 | 8 صفحه PDF | دانلود رایگان |
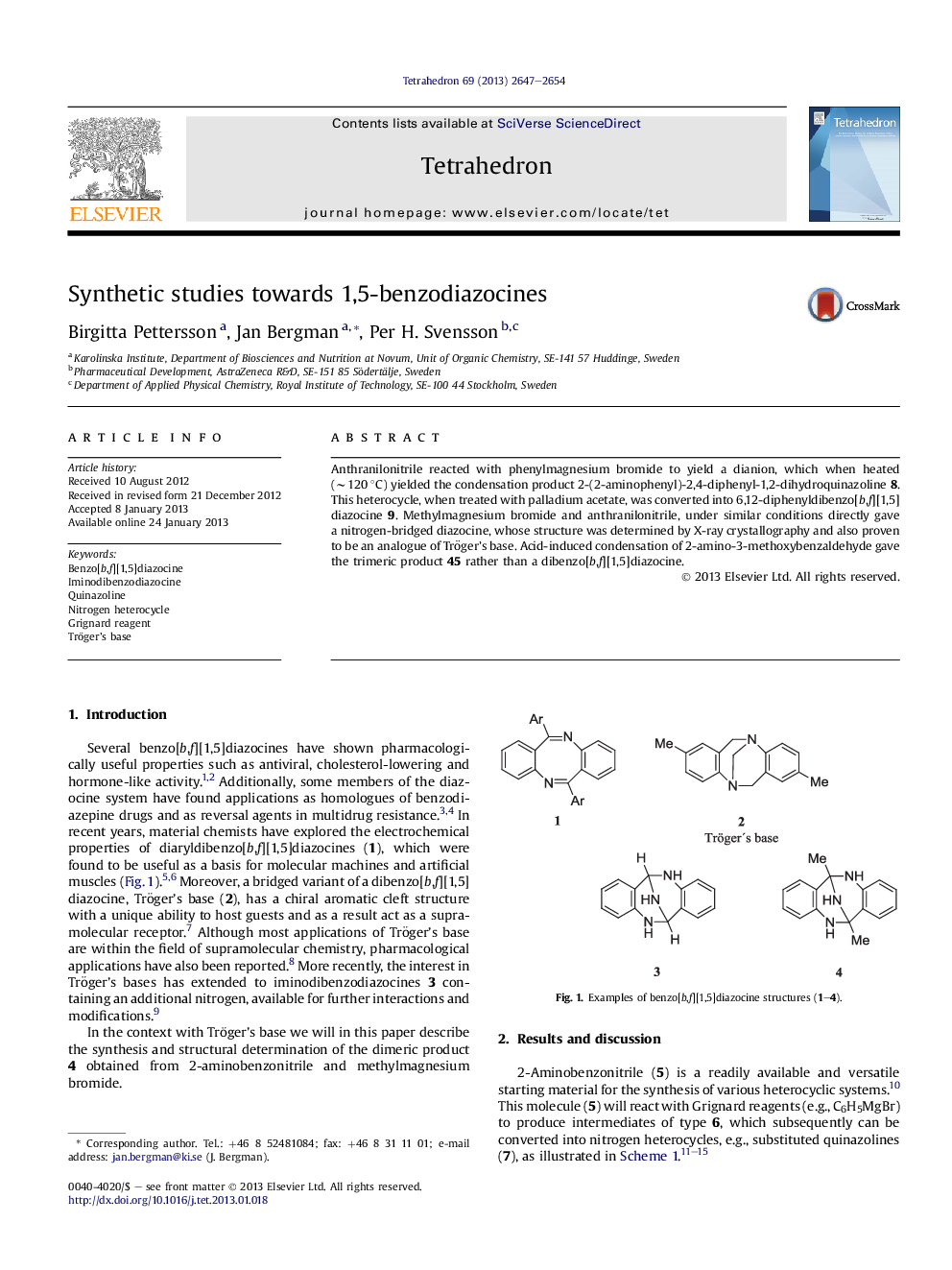
Anthranilonitrile reacted with phenylmagnesium bromide to yield a dianion, which when heated (∼120 °C) yielded the condensation product 2-(2-aminophenyl)-2,4-diphenyl-1,2-dihydroquinazoline 8. This heterocycle, when treated with palladium acetate, was converted into 6,12-diphenyldibenzo[b,f][1,5]diazocine 9. Methylmagnesium bromide and anthranilonitrile, under similar conditions directly gave a nitrogen-bridged diazocine, whose structure was determined by X-ray crystallography and also proven to be an analogue of Tröger’s base. Acid-induced condensation of 2-amino-3-methoxybenzaldehyde gave the trimeric product 45 rather than a dibenzo[b,f][1,5]diazocine.
The dianion, formed by addition of Grignard reagents to anthranilonitrile provides a rapid entry to quinazolines and benzodiazepines. Additionally, this dianion can be converted into NH-bridged 1,5-benzodiazocines.Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 69, Issue 12, 25 March 2013, Pages 2647–2654