| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5218347 | 1383324 | 2012 | 7 صفحه PDF | دانلود رایگان |
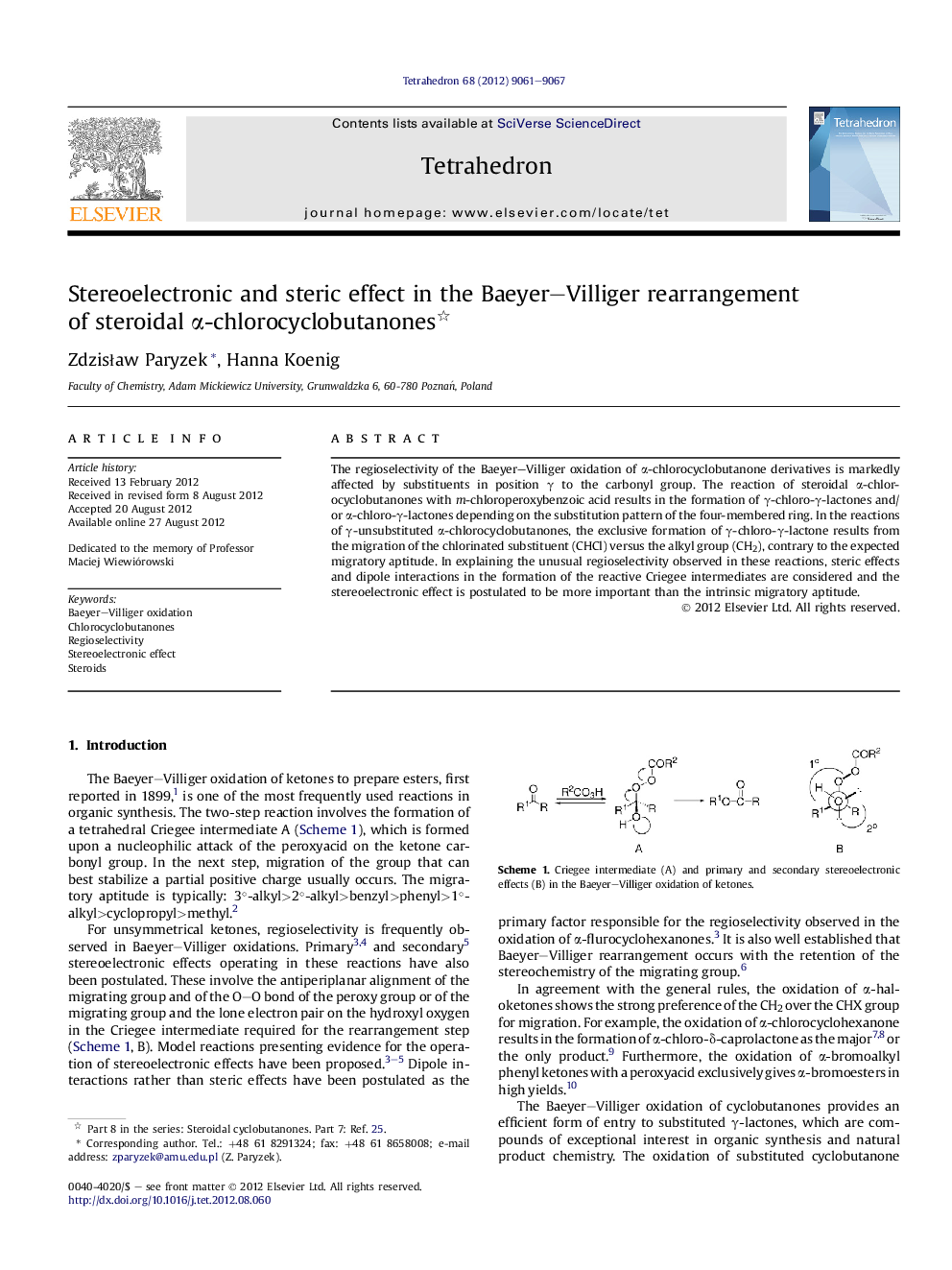
The regioselectivity of the Baeyer-Villiger oxidation of α-chlorocyclobutanone derivatives is markedly affected by substituents in position γ to the carbonyl group. The reaction of steroidal α-chlorocyclobutanones with m-chloroperoxybenzoic acid results in the formation of γ-chloro-γ-lactones and/or α-chloro-γ-lactones depending on the substitution pattern of the four-membered ring. In the reactions of γ-unsubstituted α-chlorocyclobutanones, the exclusive formation of γ-chloro-γ-lactone results from the migration of the chlorinated substituent (CHCl) versus the alkyl group (CH2), contrary to the expected migratory aptitude. In explaining the unusual regioselectivity observed in these reactions, steric effects and dipole interactions in the formation of the reactive Criegee intermediates are considered and the stereoelectronic effect is postulated to be more important than the intrinsic migratory aptitude.
Journal: Tetrahedron - Volume 68, Issue 44, 4 November 2012, Pages 9061-9067