| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5218527 | 1383330 | 2012 | 6 صفحه PDF | دانلود رایگان |
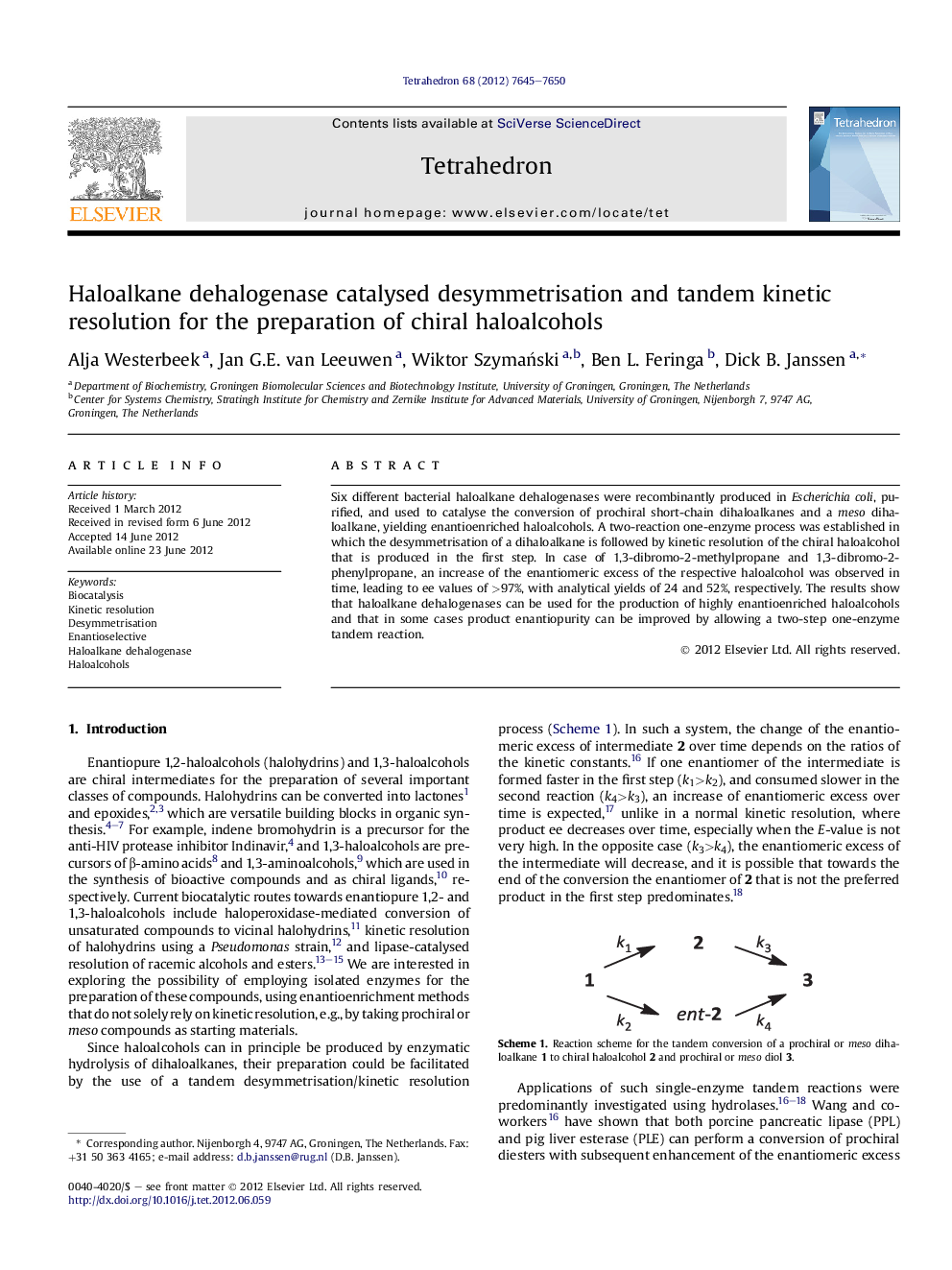
Six different bacterial haloalkane dehalogenases were recombinantly produced in Escherichia coli, purified, and used to catalyse the conversion of prochiral short-chain dihaloalkanes and a meso dihaloalkane, yielding enantioenriched haloalcohols. A two-reaction one-enzyme process was established in which the desymmetrisation of a dihaloalkane is followed by kinetic resolution of the chiral haloalcohol that is produced in the first step. In case of 1,3-dibromo-2-methylpropane and 1,3-dibromo-2-phenylpropane, an increase of the enantiomeric excess of the respective haloalcohol was observed in time, leading to ee values of >97%, with analytical yields of 24 and 52%, respectively. The results show that haloalkane dehalogenases can be used for the production of highly enantioenriched haloalcohols and that in some cases product enantiopurity can be improved by allowing a two-step one-enzyme tandem reaction.
Journal: Tetrahedron - Volume 68, Issue 37, 16 September 2012, Pages 7645-7650