| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5218948 | 1383342 | 2012 | 9 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
A flexible enantioselective approach to 3,4-dihydroxyprolinol derivatives by SmI2-mediated reductive coupling of chiral nitrone with ketones/aldehydes
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
موضوعات مرتبط
مهندسی و علوم پایه
شیمی
شیمی آلی
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
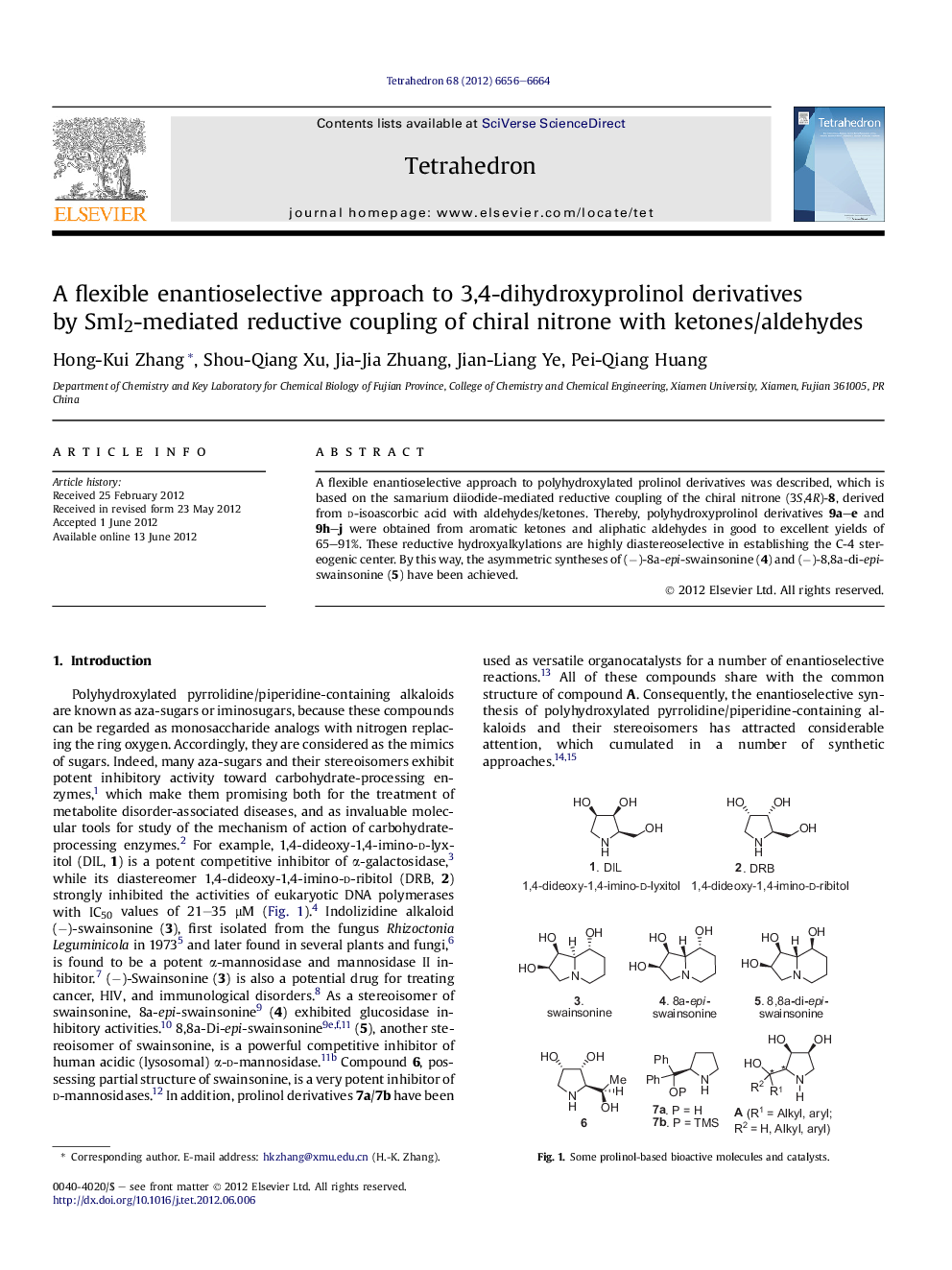
A flexible enantioselective approach to polyhydroxylated prolinol derivatives was described, which is based on the samarium diiodide-mediated reductive coupling of the chiral nitrone (3S,4R)-8, derived from d-isoascorbic acid with aldehydes/ketones. Thereby, polyhydroxyprolinol derivatives 9a-e and 9h-j were obtained from aromatic ketones and aliphatic aldehydes in good to excellent yields of 65-91%. These reductive hydroxyalkylations are highly diastereoselective in establishing the C-4 stereogenic center. By this way, the asymmetric syntheses of (â)-8a-epi-swainsonine (4) and (â)-8,8a-di-epi-swainsonine (5) have been achieved.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Tetrahedron - Volume 68, Issue 33, 19 August 2012, Pages 6656-6664
Journal: Tetrahedron - Volume 68, Issue 33, 19 August 2012, Pages 6656-6664
نویسندگان
Hong-Kui Zhang, Shou-Qiang Xu, Jia-Jia Zhuang, Jian-Liang Ye, Pei-Qiang Huang,