| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5219325 | 1383353 | 2011 | 8 صفحه PDF | دانلود رایگان |
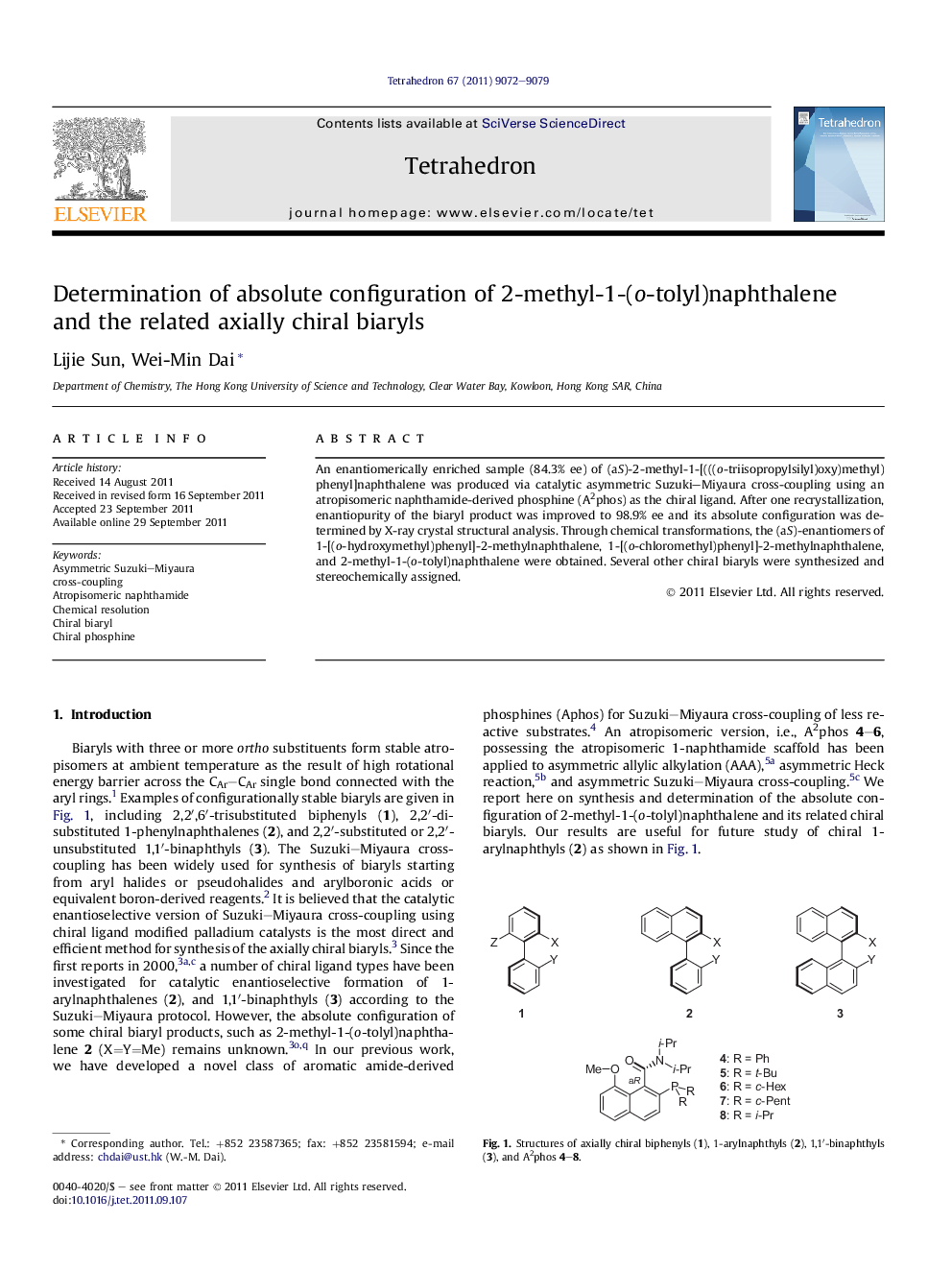
An enantiomerically enriched sample (84.3% ee) of (aS)-2-methyl-1-[(((o-triisopropylsilyl)oxy)methyl)phenyl]naphthalene was produced via catalytic asymmetric Suzuki–Miyaura cross-coupling using an atropisomeric naphthamide-derived phosphine (A2phos) as the chiral ligand. After one recrystallization, enantiopurity of the biaryl product was improved to 98.9% ee and its absolute configuration was determined by X-ray crystal structural analysis. Through chemical transformations, the (aS)-enantiomers of 1-[(o-hydroxymethyl)phenyl]-2-methylnaphthalene, 1-[(o-chloromethyl)phenyl]-2-methylnaphthalene, and 2-methyl-1-(o-tolyl)naphthalene were obtained. Several other chiral biaryls were synthesized and stereochemically assigned.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 67, Issue 47, 25 November 2011, Pages 9072–9079