| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5219338 | 1383353 | 2011 | 6 صفحه PDF | دانلود رایگان |
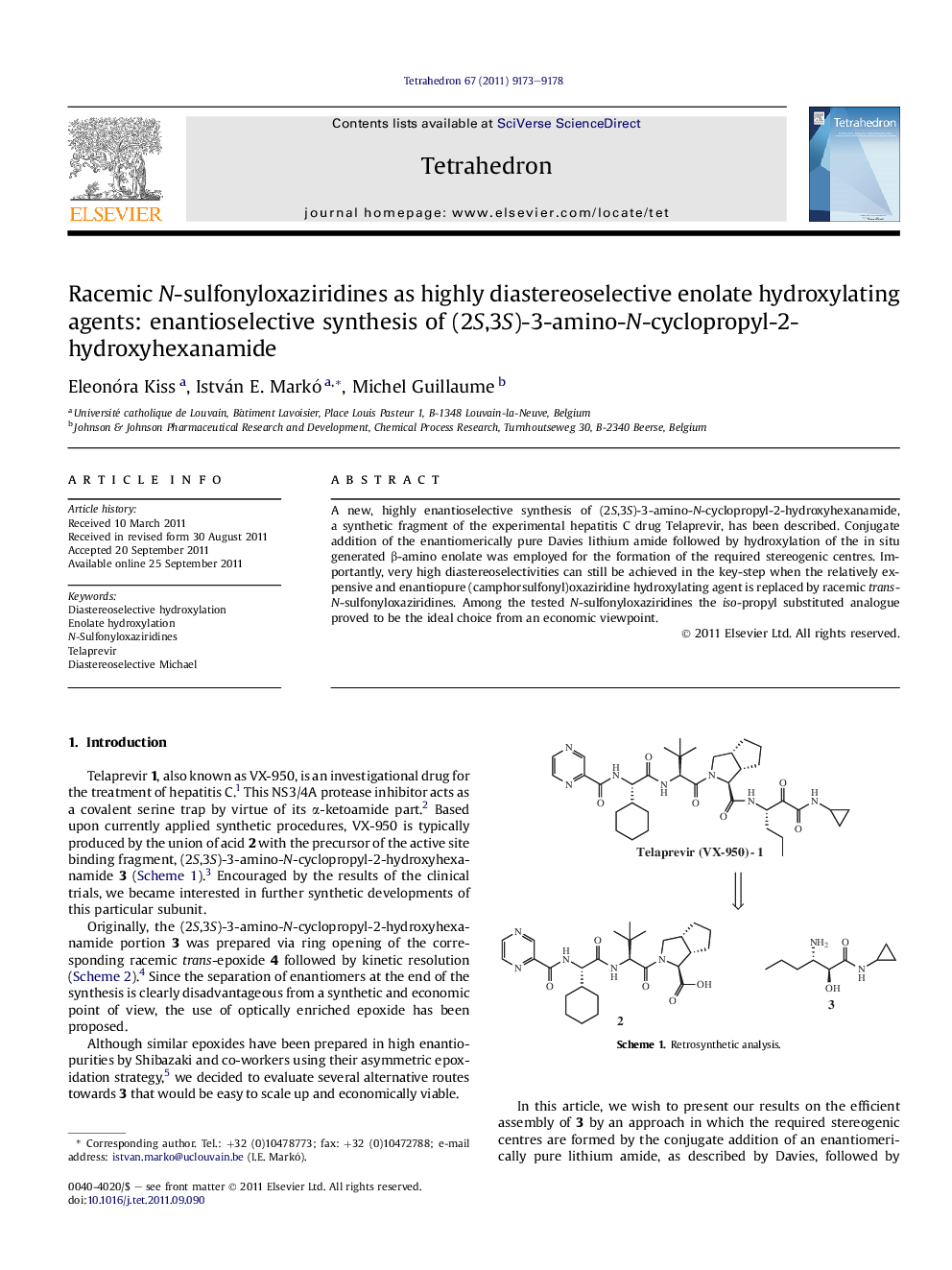
A new, highly enantioselective synthesis of (2S,3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide, a synthetic fragment of the experimental hepatitis C drug Telaprevir, has been described. Conjugate addition of the enantiomerically pure Davies lithium amide followed by hydroxylation of the in situ generated β-amino enolate was employed for the formation of the required stereogenic centres. Importantly, very high diastereoselectivities can still be achieved in the key-step when the relatively expensive and enantiopure (camphorsulfonyl)oxaziridine hydroxylating agent is replaced by racemic trans-N-sulfonyloxaziridines. Among the tested N-sulfonyloxaziridines the iso-propyl substituted analogue proved to be the ideal choice from an economic viewpoint.
Journal: Tetrahedron - Volume 67, Issue 47, 25 November 2011, Pages 9173-9178