| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5219421 | 1383356 | 2012 | 5 صفحه PDF | دانلود رایگان |
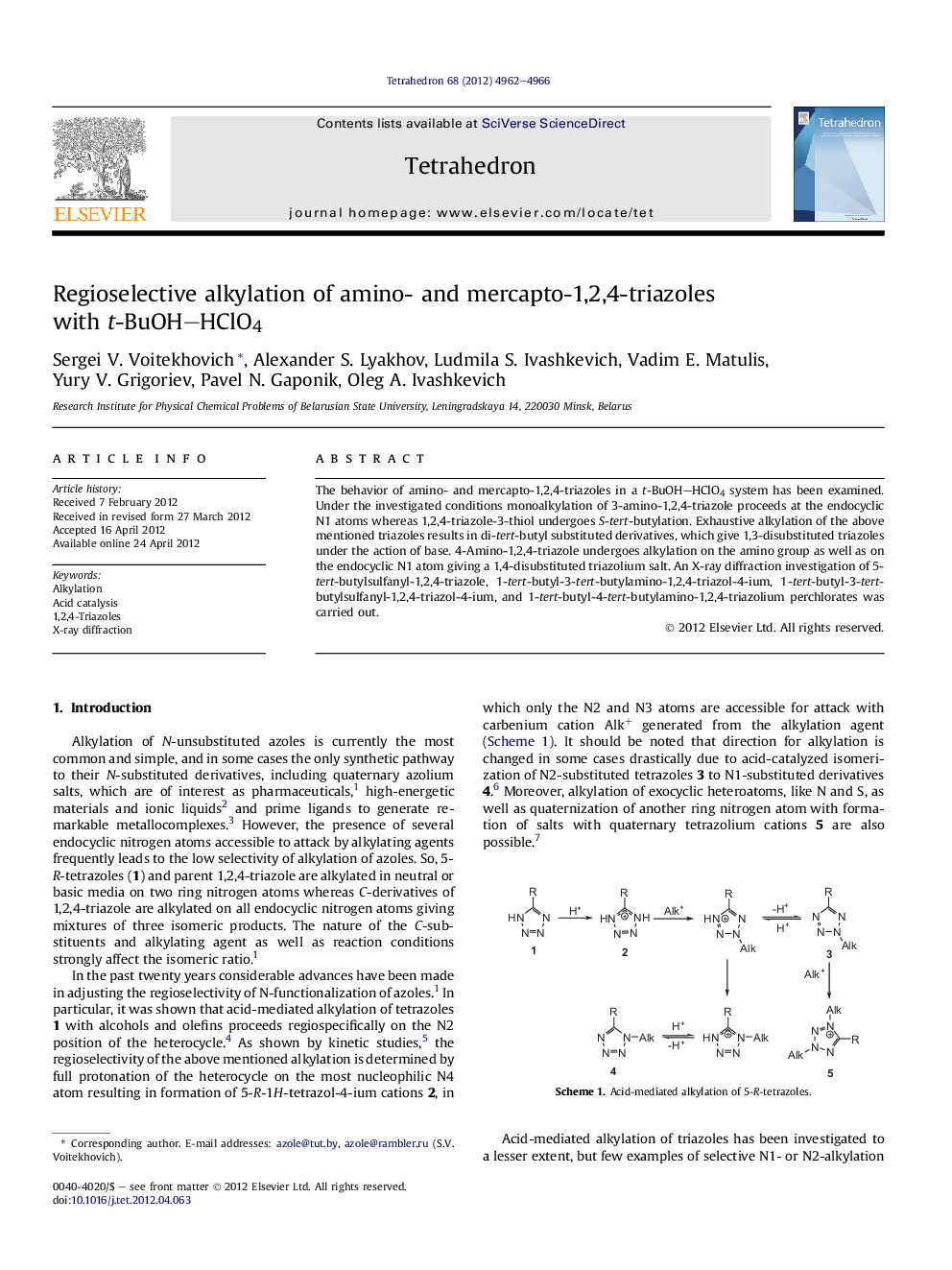
The behavior of amino- and mercapto-1,2,4-triazoles in a t-BuOH–HClO4 system has been examined. Under the investigated conditions monoalkylation of 3-amino-1,2,4-triazole proceeds at the endocyclic N1 atoms whereas 1,2,4-triazole-3-thiol undergoes S-tert-butylation. Exhaustive alkylation of the above mentioned triazoles results in di-tert-butyl substituted derivatives, which give 1,3-disubstituted triazoles under the action of base. 4-Amino-1,2,4-triazole undergoes alkylation on the amino group as well as on the endocyclic N1 atom giving a 1,4-disubstituted triazolium salt. An X-ray diffraction investigation of 5-tert-butylsulfanyl-1,2,4-triazole, 1-tert-butyl-3-tert-butylamino-1,2,4-triazol-4-ium, 1-tert-butyl-3-tert-butylsulfanyl-1,2,4-triazol-4-ium, and 1-tert-butyl-4-tert-butylamino-1,2,4-triazolium perchlorates was carried out.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 68, Issue 25, 24 June 2012, Pages 4962–4966