| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5219723 | 1383366 | 2012 | 9 صفحه PDF | دانلود رایگان |
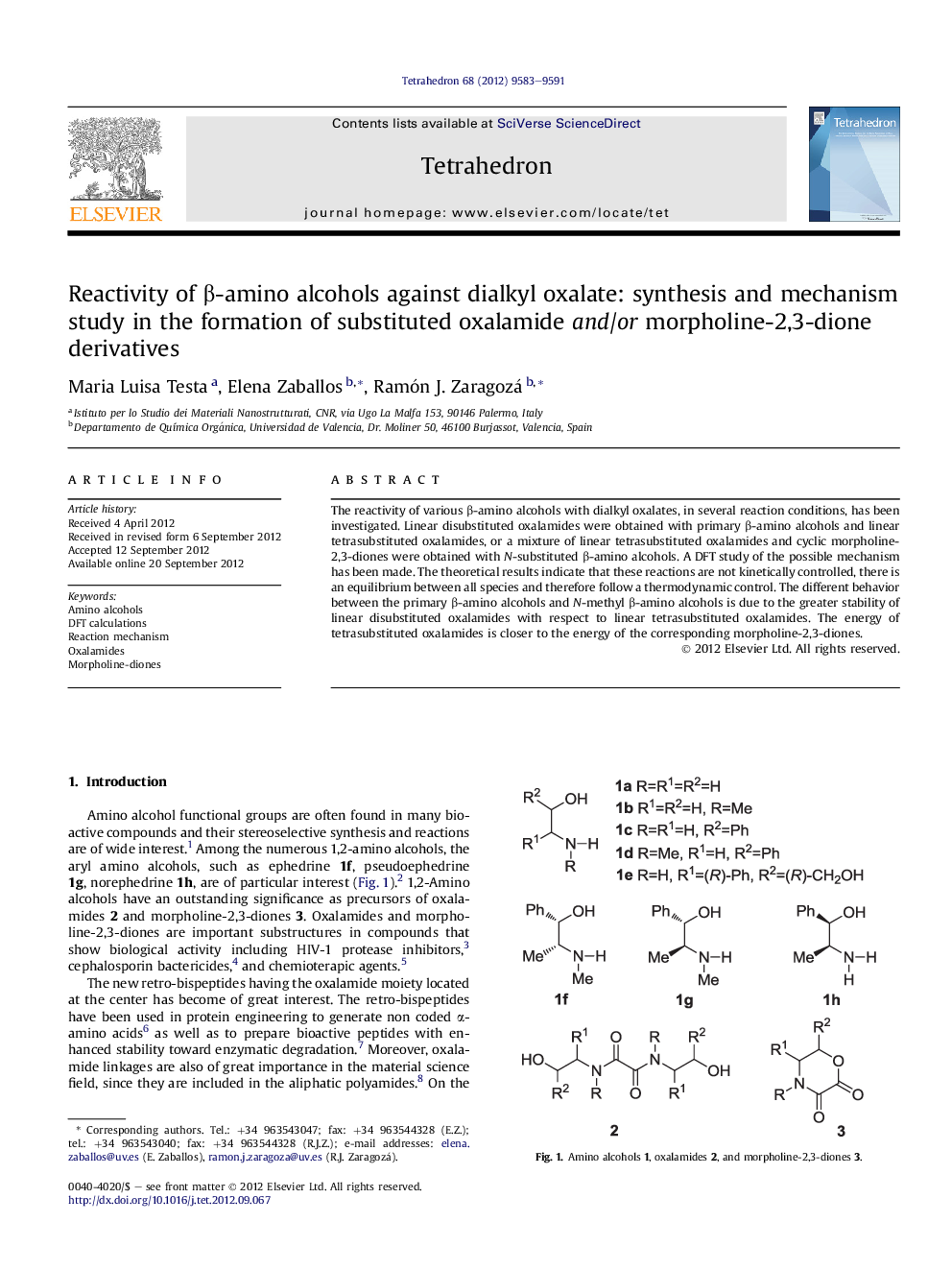
The reactivity of various β-amino alcohols with dialkyl oxalates, in several reaction conditions, has been investigated. Linear disubstituted oxalamides were obtained with primary β-amino alcohols and linear tetrasubstituted oxalamides, or a mixture of linear tetrasubstituted oxalamides and cyclic morpholine-2,3-diones were obtained with N-substituted β-amino alcohols. A DFT study of the possible mechanism has been made. The theoretical results indicate that these reactions are not kinetically controlled, there is an equilibrium between all species and therefore follow a thermodynamic control. The different behavior between the primary β-amino alcohols and N-methyl β-amino alcohols is due to the greater stability of linear disubstituted oxalamides with respect to linear tetrasubstituted oxalamides. The energy of tetrasubstituted oxalamides is closer to the energy of the corresponding morpholine-2,3-diones.
An experimental and theoretical study of the reactivity of various β-amino alcohols 1 with oxalates indicates that there is an equilibrium between all species and therefore the reaction follows a thermodynamic control.Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 68, Issue 47, 25 November 2012, Pages 9583–9591