| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5219831 | 1383369 | 2012 | 9 صفحه PDF | دانلود رایگان |
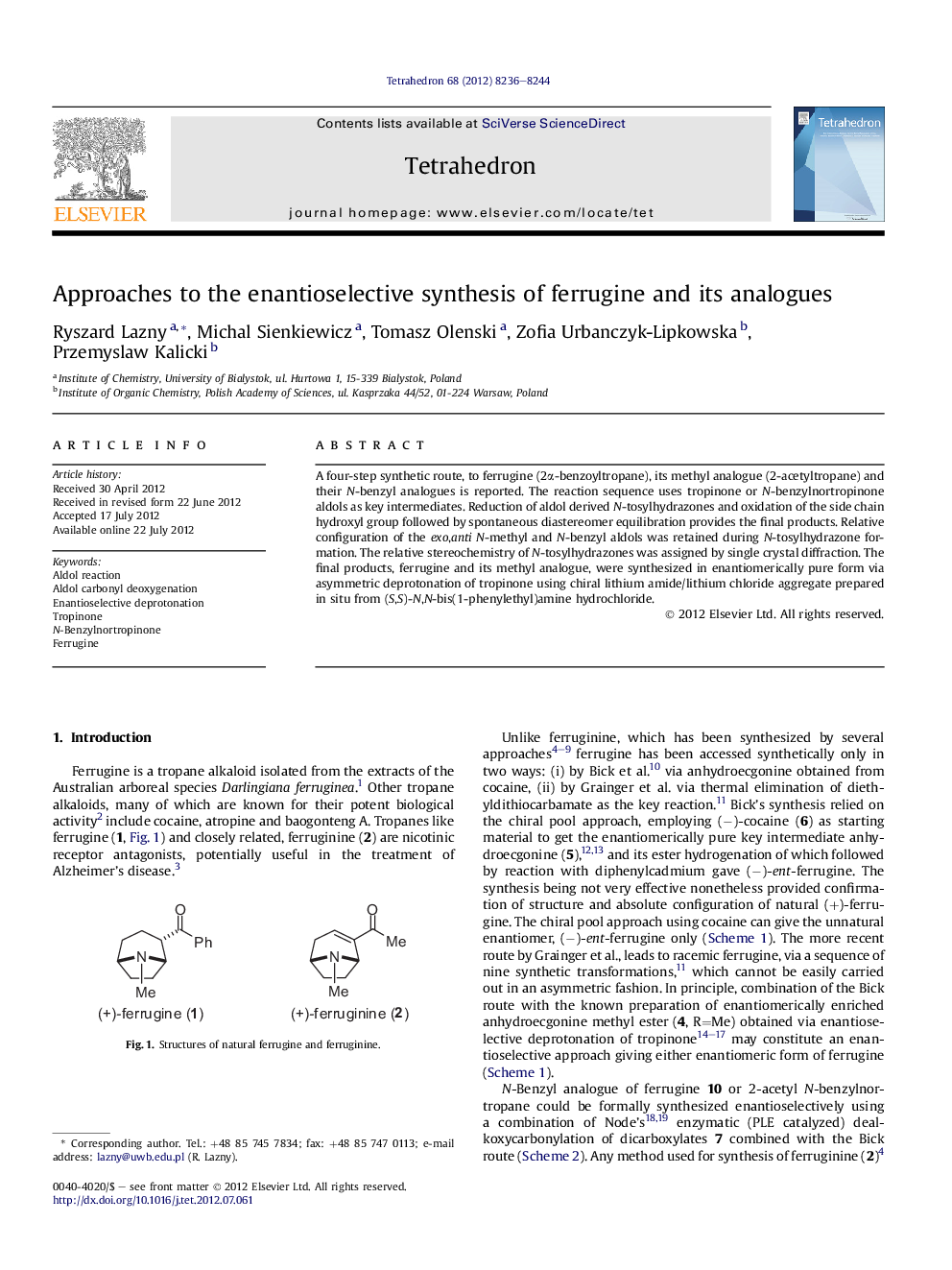
A four-step synthetic route, to ferrugine (2α-benzoyltropane), its methyl analogue (2-acetyltropane) and their N-benzyl analogues is reported. The reaction sequence uses tropinone or N-benzylnortropinone aldols as key intermediates. Reduction of aldol derived N-tosylhydrazones and oxidation of the side chain hydroxyl group followed by spontaneous diastereomer equilibration provides the final products. Relative configuration of the exo,anti N-methyl and N-benzyl aldols was retained during N-tosylhydrazone formation. The relative stereochemistry of N-tosylhydrazones was assigned by single crystal diffraction. The final products, ferrugine and its methyl analogue, were synthesized in enantiomerically pure form via asymmetric deprotonation of tropinone using chiral lithium amide/lithium chloride aggregate prepared in situ from (S,S)-N,N-bis(1-phenylethyl)amine hydrochloride.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 68, Issue 39, 30 September 2012, Pages 8236–8244