| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5220185 | 1383380 | 2011 | 10 صفحه PDF | دانلود رایگان |
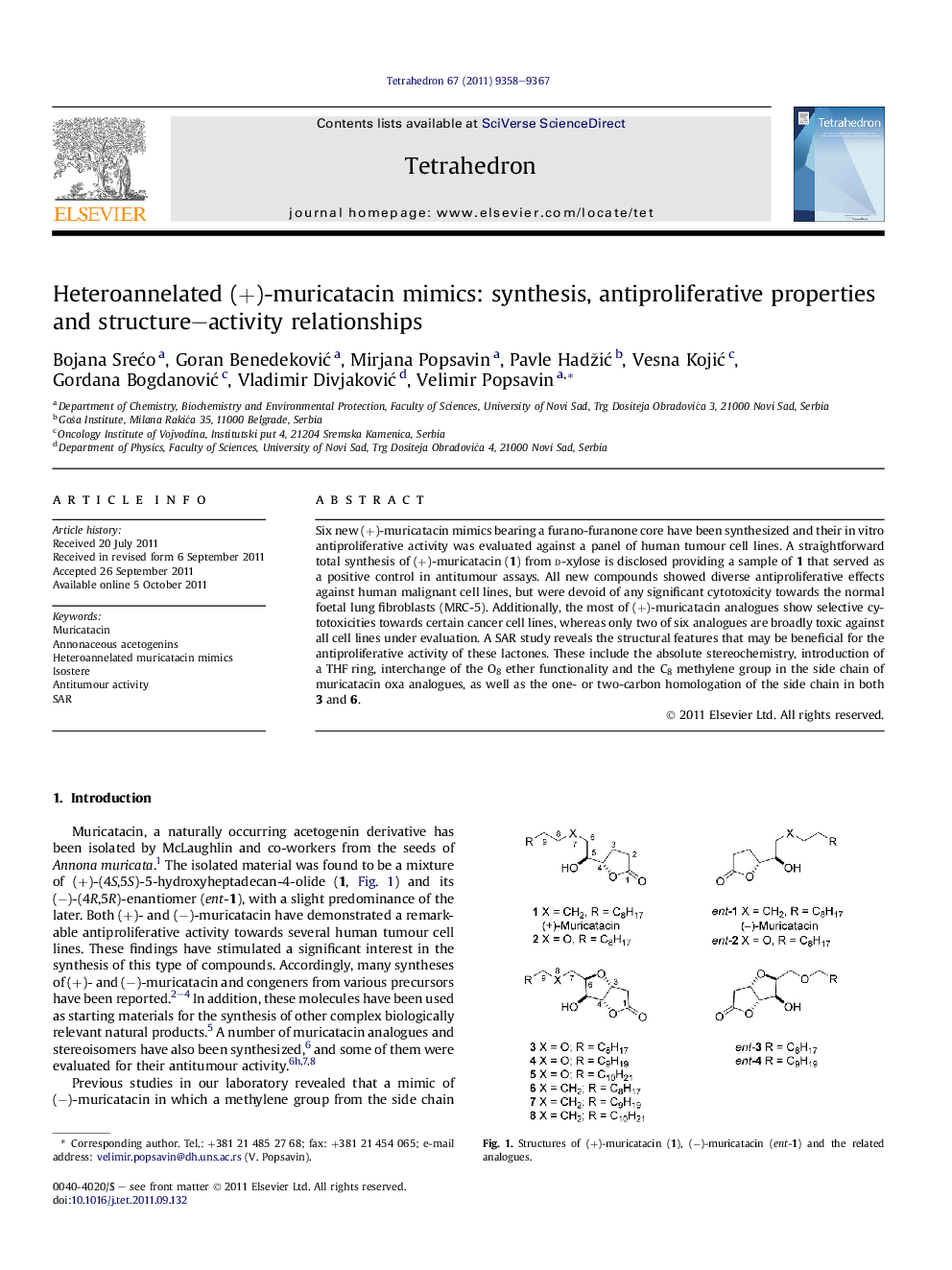
Six new (+)-muricatacin mimics bearing a furano-furanone core have been synthesized and their in vitro antiproliferative activity was evaluated against a panel of human tumour cell lines. A straightforward total synthesis of (+)-muricatacin (1) from d-xylose is disclosed providing a sample of 1 that served as a positive control in antitumour assays. All new compounds showed diverse antiproliferative effects against human malignant cell lines, but were devoid of any significant cytotoxicity towards the normal foetal lung fibroblasts (MRC-5). Additionally, the most of (+)-muricatacin analogues show selective cytotoxicities towards certain cancer cell lines, whereas only two of six analogues are broadly toxic against all cell lines under evaluation. A SAR study reveals the structural features that may be beneficial for the antiproliferative activity of these lactones. These include the absolute stereochemistry, introduction of a THF ring, interchange of the O8 ether functionality and the C8 methylene group in the side chain of muricatacin oxa analogues, as well as the one- or two-carbon homologation of the side chain in both 3 and 6.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 67, Issue 48, 2 December 2011, Pages 9358–9367