| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5220574 | 1383392 | 2012 | 8 صفحه PDF | دانلود رایگان |
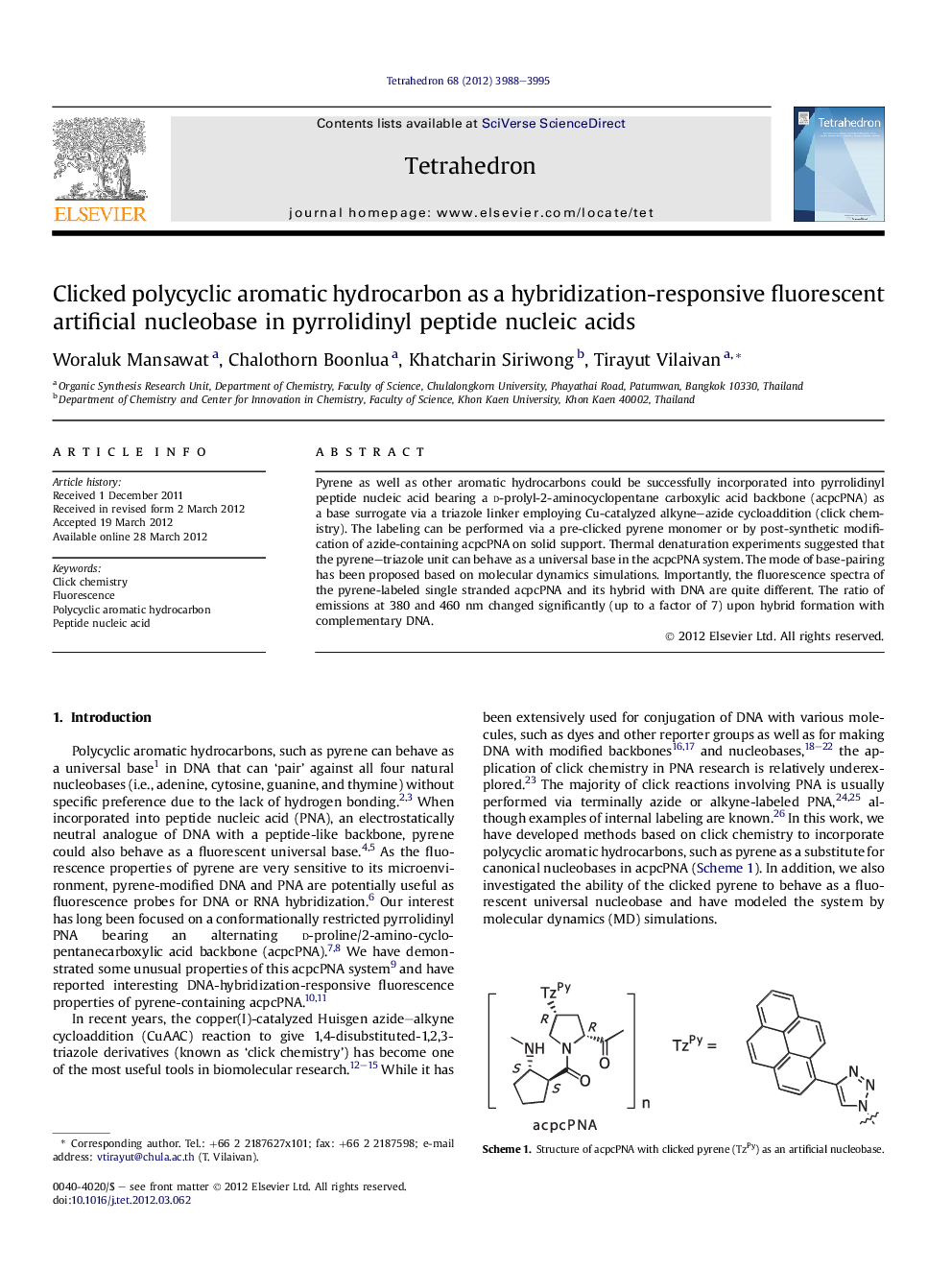
Pyrene as well as other aromatic hydrocarbons could be successfully incorporated into pyrrolidinyl peptide nucleic acid bearing a d-prolyl-2-aminocyclopentane carboxylic acid backbone (acpcPNA) as a base surrogate via a triazole linker employing Cu-catalyzed alkyne–azide cycloaddition (click chemistry). The labeling can be performed via a pre-clicked pyrene monomer or by post-synthetic modification of azide-containing acpcPNA on solid support. Thermal denaturation experiments suggested that the pyrene–triazole unit can behave as a universal base in the acpcPNA system. The mode of base-pairing has been proposed based on molecular dynamics simulations. Importantly, the fluorescence spectra of the pyrene-labeled single stranded acpcPNA and its hybrid with DNA are quite different. The ratio of emissions at 380 and 460 nm changed significantly (up to a factor of 7) upon hybrid formation with complementary DNA.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 68, Issue 21, 27 May 2012, Pages 3988–3995