| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5220695 | 1383396 | 2011 | 8 صفحه PDF | دانلود رایگان |
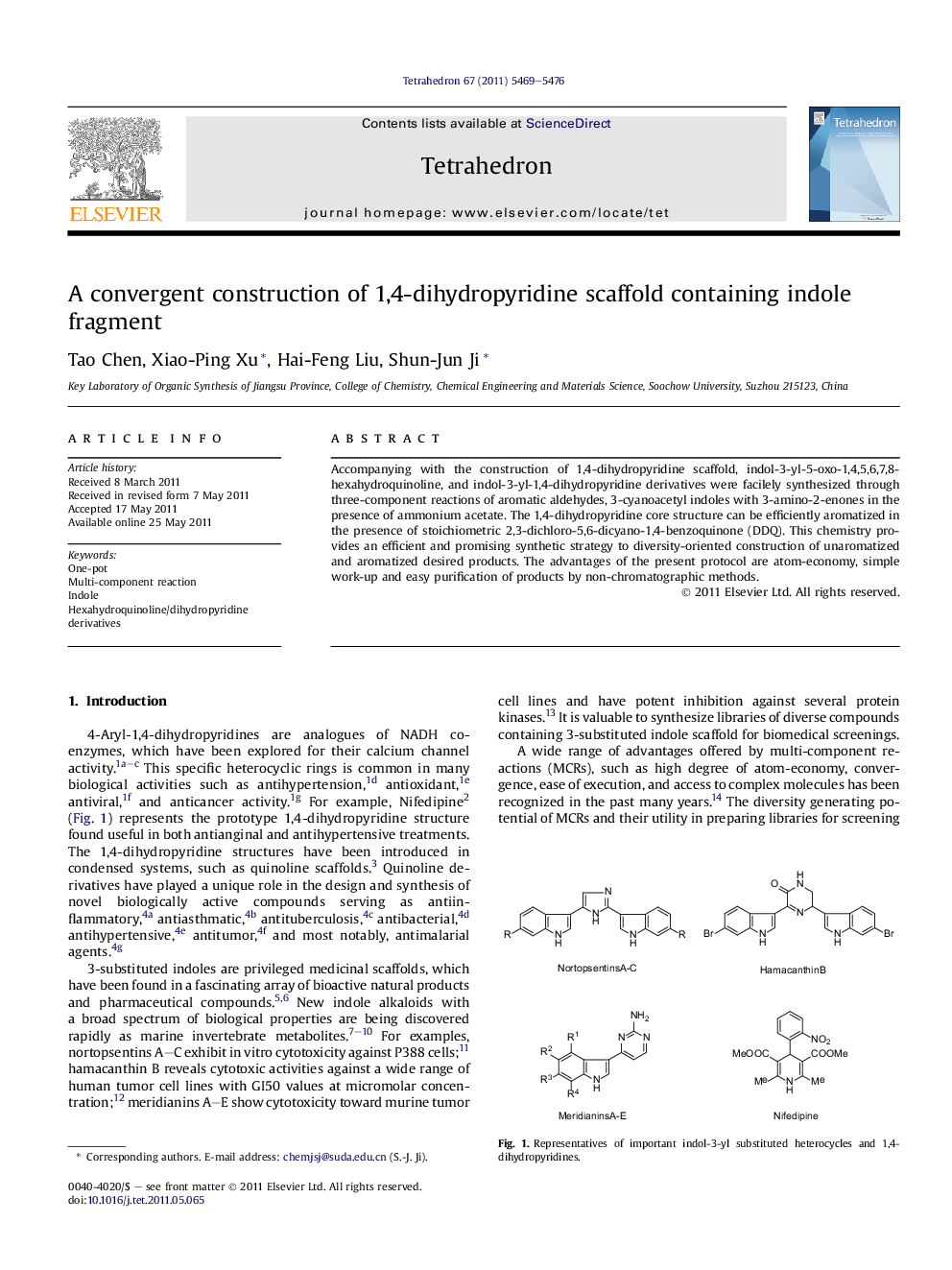
Accompanying with the construction of 1,4-dihydropyridine scaffold, indol-3-yl-5-oxo-1,4,5,6,7,8-hexahydroquinoline, and indol-3-yl-1,4-dihydropyridine derivatives were facilely synthesized through three-component reactions of aromatic aldehydes, 3-cyanoacetyl indoles with 3-amino-2-enones in the presence of ammonium acetate. The 1,4-dihydropyridine core structure can be efficiently aromatized in the presence of stoichiometric 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). This chemistry provides an efficient and promising synthetic strategy to diversity-oriented construction of unaromatized and aromatized desired products. The advantages of the present protocol are atom-economy, simple work-up and easy purification of products by non-chromatographic methods.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 67, Issue 30, 29 July 2011, Pages 5469–5476