| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5221084 | 1383408 | 2010 | 7 صفحه PDF | دانلود رایگان |
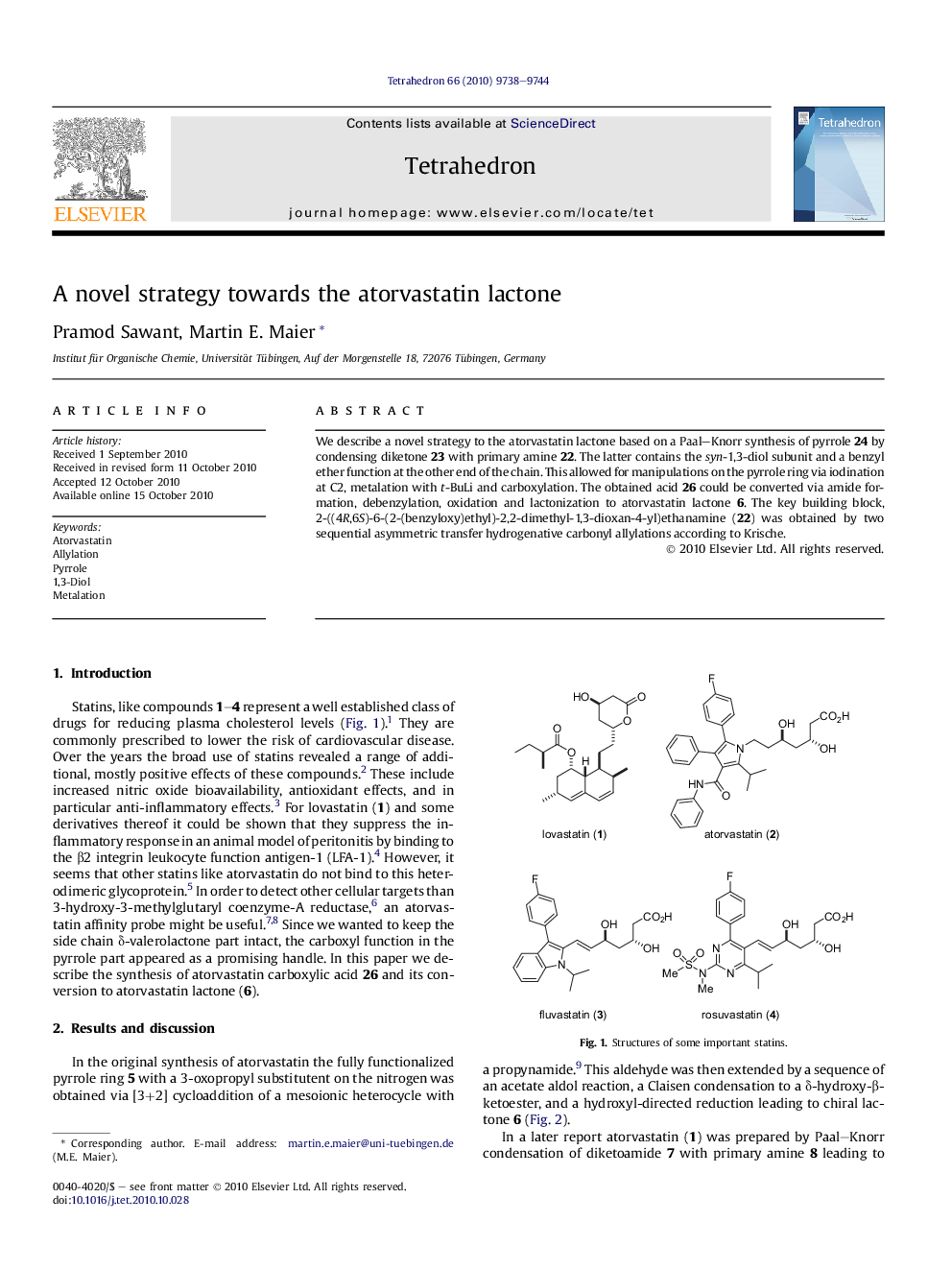
We describe a novel strategy to the atorvastatin lactone based on a Paal–Knorr synthesis of pyrrole 24 by condensing diketone 23 with primary amine 22. The latter contains the syn-1,3-diol subunit and a benzyl ether function at the other end of the chain. This allowed for manipulations on the pyrrole ring via iodination at C2, metalation with t-BuLi and carboxylation. The obtained acid 26 could be converted via amide formation, debenzylation, oxidation and lactonization to atorvastatin lactone 6. The key building block, 2-((4R,6S)-6-(2-(benzyloxy)ethyl)-2,2-dimethyl-1,3-dioxan-4-yl)ethanamine (22) was obtained by two sequential asymmetric transfer hydrogenative carbonyl allylations according to Krische.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 66, Issue 51, 18 December 2010, Pages 9738–9744