| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5222013 | 1383439 | 2009 | 13 صفحه PDF | دانلود رایگان |
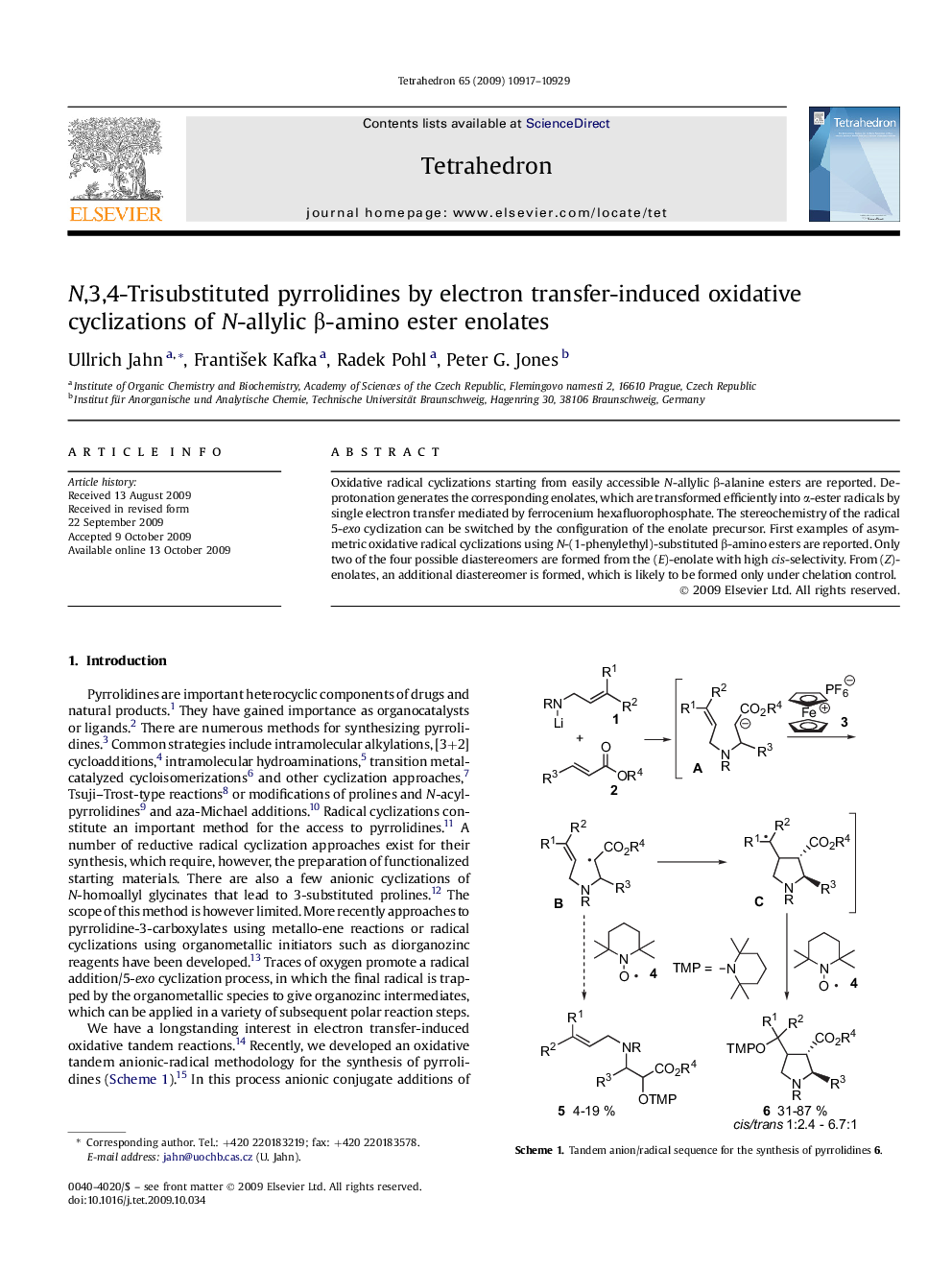
Oxidative radical cyclizations starting from easily accessible N-allylic β-alanine esters are reported. Deprotonation generates the corresponding enolates, which are transformed efficiently into α-ester radicals by single electron transfer mediated by ferrocenium hexafluorophosphate. The stereochemistry of the radical 5-exo cyclization can be switched by the configuration of the enolate precursor. First examples of asymmetric oxidative radical cyclizations using N-(1-phenylethyl)-substituted β-amino esters are reported. Only two of the four possible diastereomers are formed from the (E)-enolate with high cis-selectivity. From (Z)-enolates, an additional diastereomer is formed, which is likely to be formed only under chelation control.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 65, Issue 52, 26 December 2009, Pages 10917–10929