| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5222049 | 1503211 | 2011 | 16 صفحه PDF | دانلود رایگان |
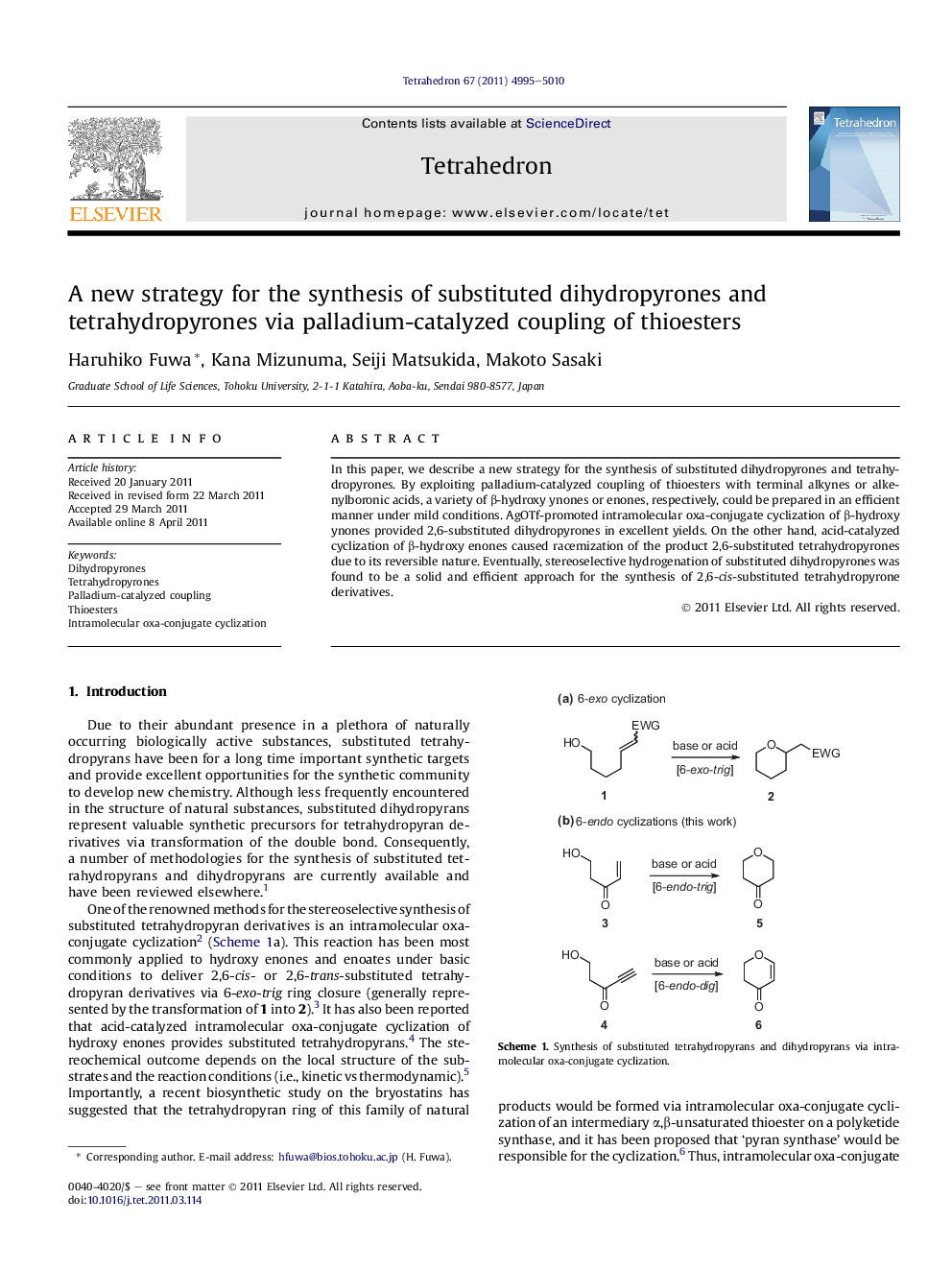
In this paper, we describe a new strategy for the synthesis of substituted dihydropyrones and tetrahydropyrones. By exploiting palladium-catalyzed coupling of thioesters with terminal alkynes or alkenylboronic acids, a variety of β-hydroxy ynones or enones, respectively, could be prepared in an efficient manner under mild conditions. AgOTf-promoted intramolecular oxa-conjugate cyclization of β-hydroxy ynones provided 2,6-substituted dihydropyrones in excellent yields. On the other hand, acid-catalyzed cyclization of β-hydroxy enones caused racemization of the product 2,6-substituted tetrahydropyrones due to its reversible nature. Eventually, stereoselective hydrogenation of substituted dihydropyrones was found to be a solid and efficient approach for the synthesis of 2,6-cis-substituted tetrahydropyrone derivatives.
Journal: Tetrahedron - Volume 67, Issues 27â28, 8 July 2011, Pages 4995-5010