| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5222069 | 1383442 | 2011 | 9 صفحه PDF | دانلود رایگان |
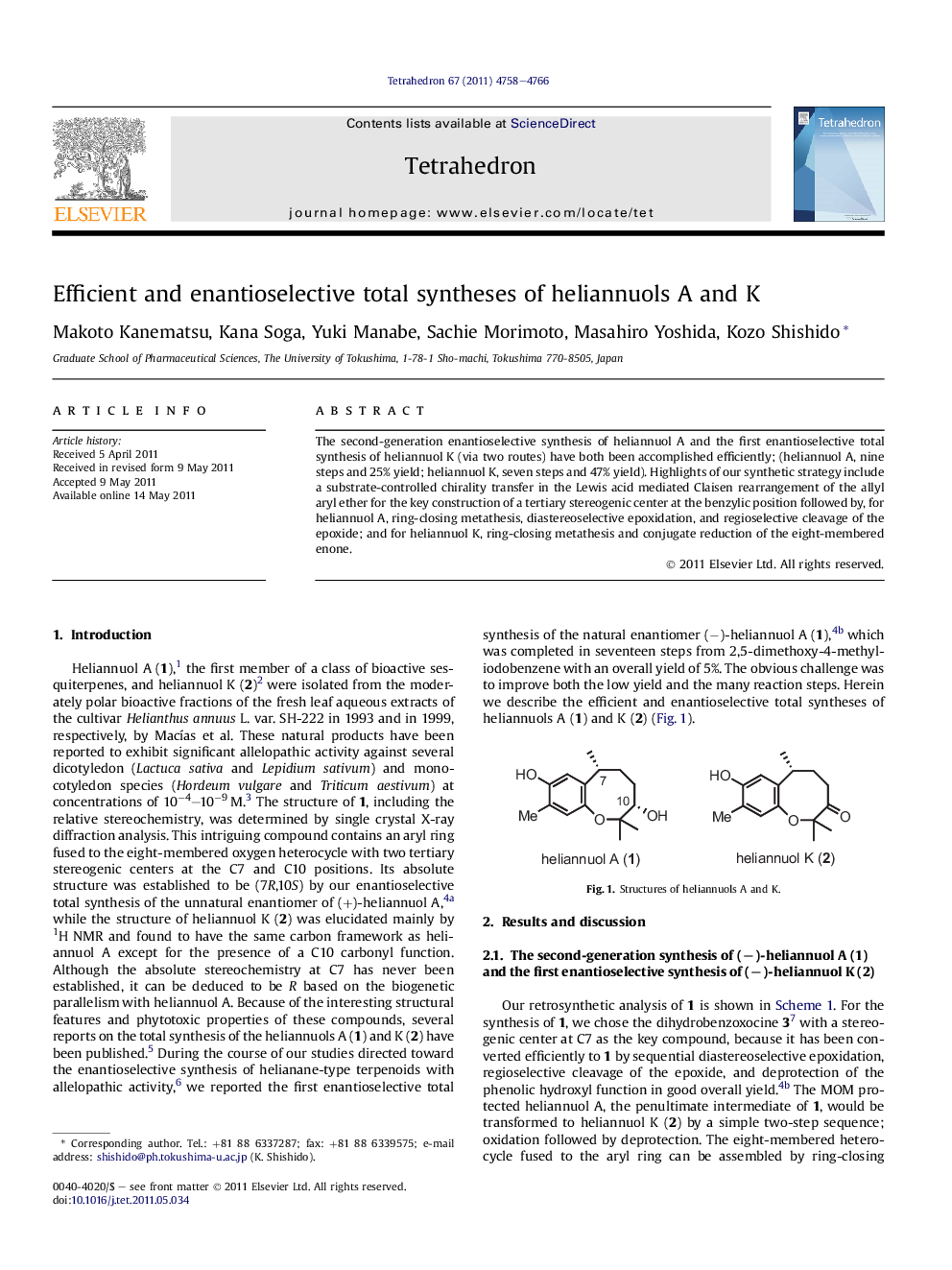
The second-generation enantioselective synthesis of heliannuol A and the first enantioselective total synthesis of heliannuol K (via two routes) have both been accomplished efficiently; (heliannuol A, nine steps and 25% yield; heliannuol K, seven steps and 47% yield). Highlights of our synthetic strategy include a substrate-controlled chirality transfer in the Lewis acid mediated Claisen rearrangement of the allyl aryl ether for the key construction of a tertiary stereogenic center at the benzylic position followed by, for heliannuol A, ring-closing metathesis, diastereoselective epoxidation, and regioselective cleavage of the epoxide; and for heliannuol K, ring-closing metathesis and conjugate reduction of the eight-membered enone.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 67, Issue 26, 1 July 2011, Pages 4758–4766