| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5222816 | 1383466 | 2009 | 9 صفحه PDF | دانلود رایگان |
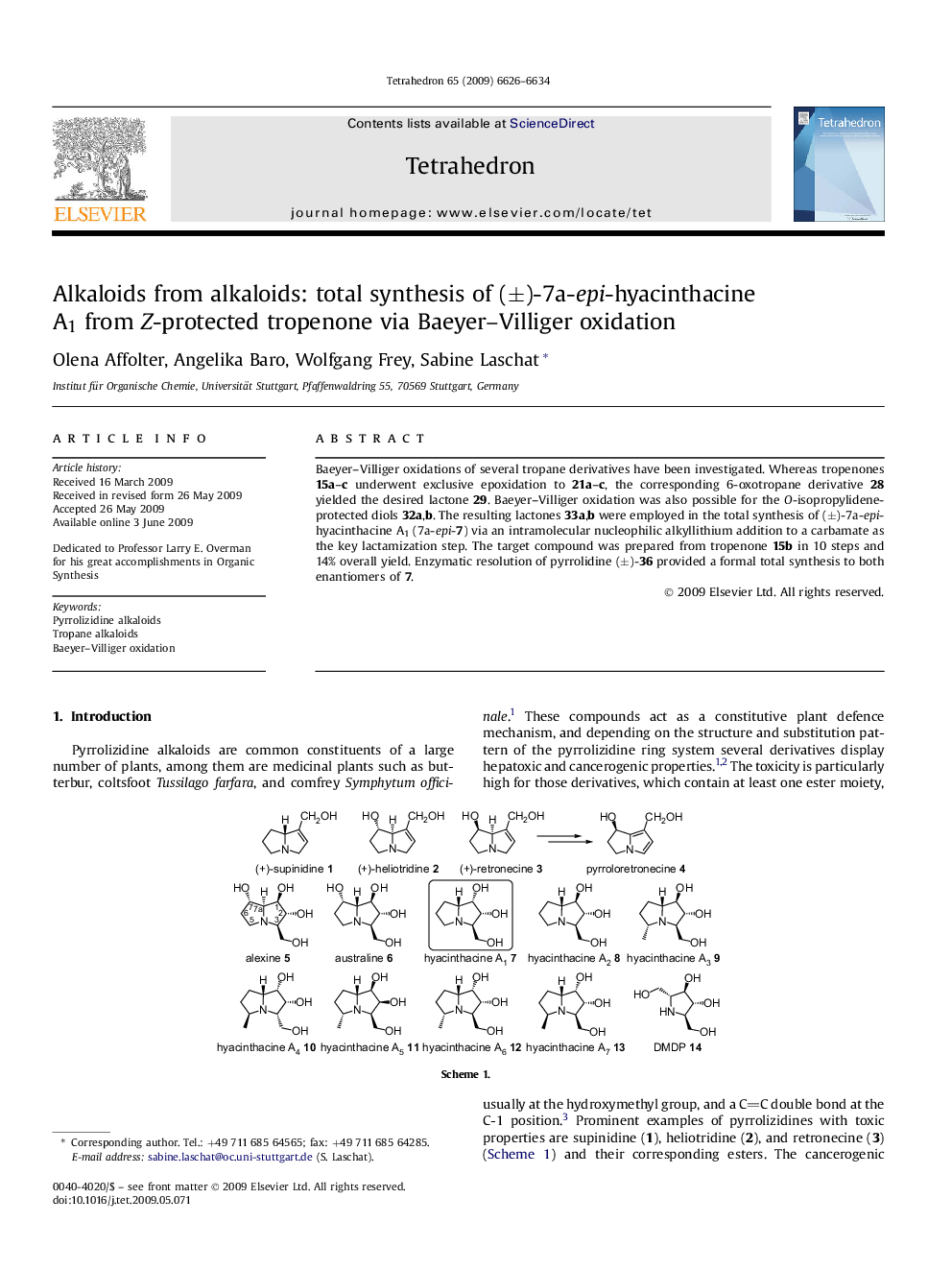
Baeyer–Villiger oxidations of several tropane derivatives have been investigated. Whereas tropenones 15a–c underwent exclusive epoxidation to 21a–c, the corresponding 6-oxotropane derivative 28 yielded the desired lactone 29. Baeyer–Villiger oxidation was also possible for the O-isopropylidene-protected diols 32a,b. The resulting lactones 33a,b were employed in the total synthesis of (±)-7a-epi-hyacinthacine A1 (7a-epi-7) via an intramolecular nucleophilic alkyllithium addition to a carbamate as the key lactamization step. The target compound was prepared from tropenone 15b in 10 steps and 14% overall yield. Enzymatic resolution of pyrrolidine (±)-36 provided a formal total synthesis to both enantiomers of 7.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron - Volume 65, Issue 33, 15 August 2009, Pages 6626–6634