| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5223689 | 1383495 | 2010 | 7 صفحه PDF | دانلود رایگان |
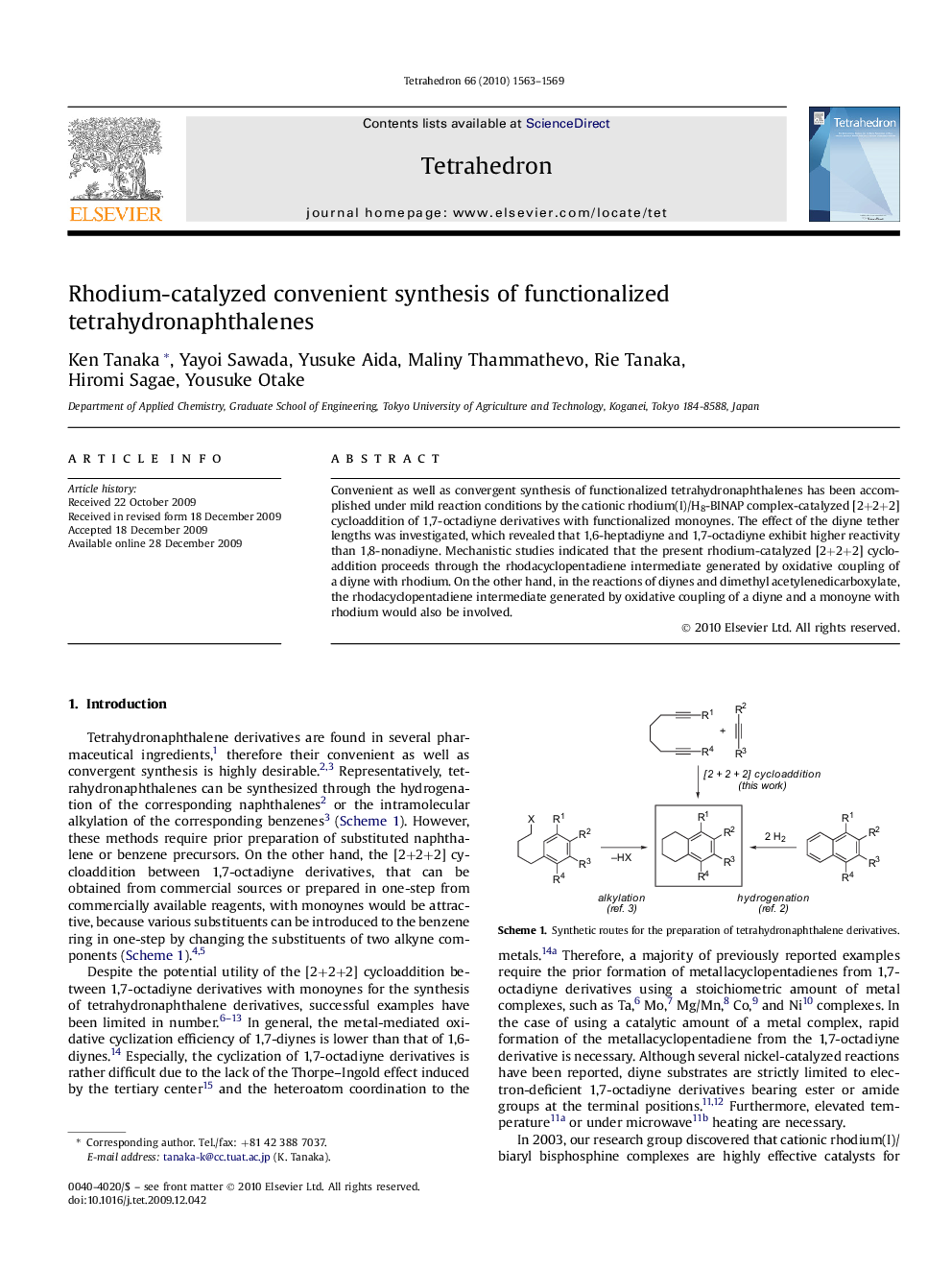
Convenient as well as convergent synthesis of functionalized tetrahydronaphthalenes has been accomplished under mild reaction conditions by the cationic rhodium(I)/H8-BINAP complex-catalyzed [2+2+2] cycloaddition of 1,7-octadiyne derivatives with functionalized monoynes. The effect of the diyne tether lengths was investigated, which revealed that 1,6-heptadiyne and 1,7-octadiyne exhibit higher reactivity than 1,8-nonadiyne. Mechanistic studies indicated that the present rhodium-catalyzed [2+2+2] cycloaddition proceeds through the rhodacyclopentadiene intermediate generated by oxidative coupling of a diyne with rhodium. On the other hand, in the reactions of diynes and dimethyl acetylenedicarboxylate, the rhodacyclopentadiene intermediate generated by oxidative coupling of a diyne and a monoyne with rhodium would also be involved.
Journal: Tetrahedron - Volume 66, Issue 8, 20 February 2010, Pages 1563-1569