| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5225364 | 1383545 | 2007 | 12 صفحه PDF | دانلود رایگان |
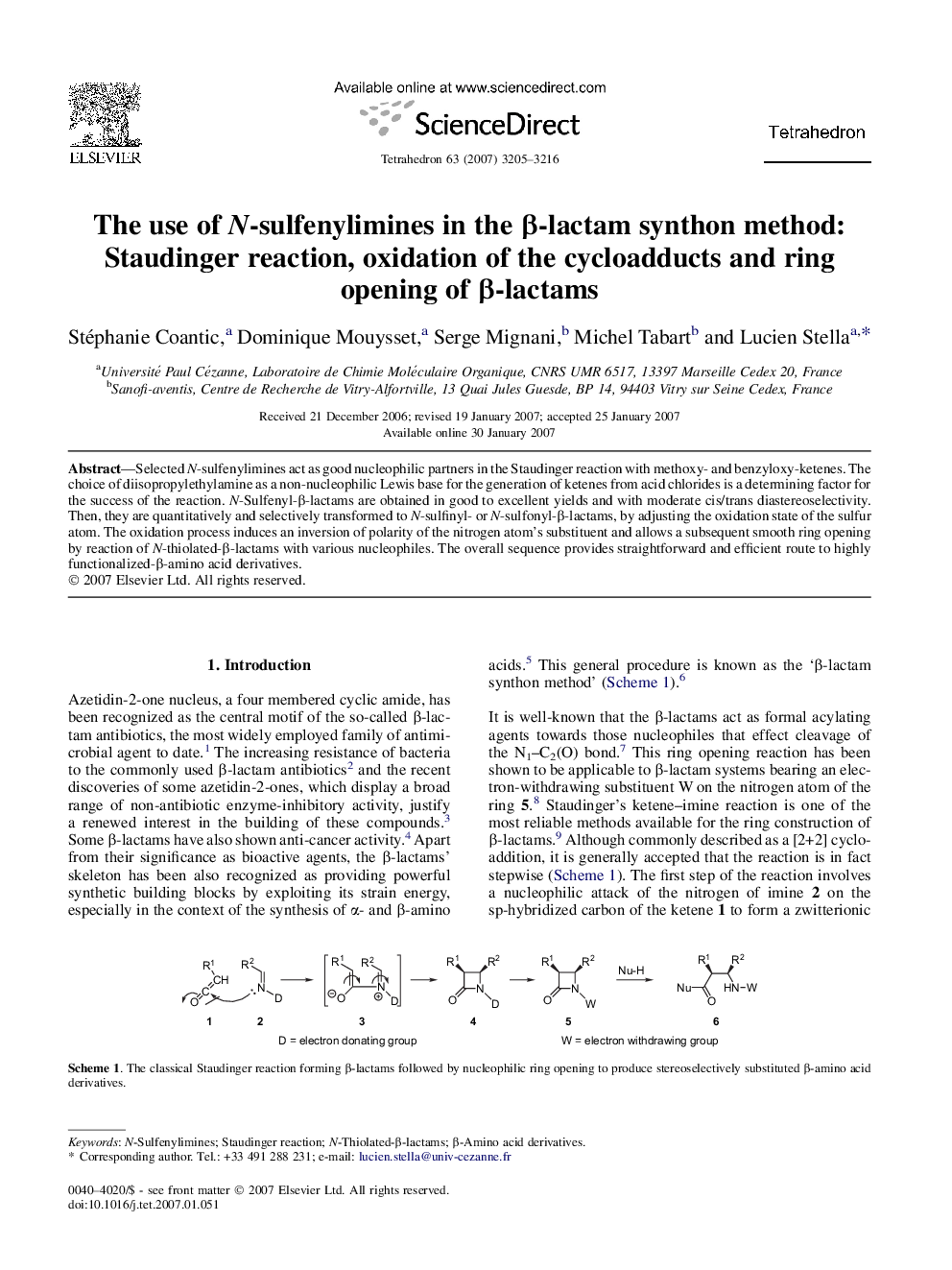
Selected N-sulfenylimines act as good nucleophilic partners in the Staudinger reaction with methoxy- and benzyloxy-ketenes. The choice of diisopropylethylamine as a non-nucleophilic Lewis base for the generation of ketenes from acid chlorides is a determining factor for the success of the reaction. N-Sulfenyl-β-lactams are obtained in good to excellent yields and with moderate cis/trans diastereoselectivity. Then, they are quantitatively and selectively transformed to N-sulfinyl- or N-sulfonyl-β-lactams, by adjusting the oxidation state of the sulfur atom. The oxidation process induces an inversion of polarity of the nitrogen atom's substituent and allows a subsequent smooth ring opening by reaction of N-thiolated-β-lactams with various nucleophiles. The overall sequence provides straightforward and efficient route to highly functionalized-β-amino acid derivatives.
Journal: Tetrahedron - Volume 63, Issue 15, 9 April 2007, Pages 3205-3216