| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5264959 | 1385271 | 2014 | 4 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
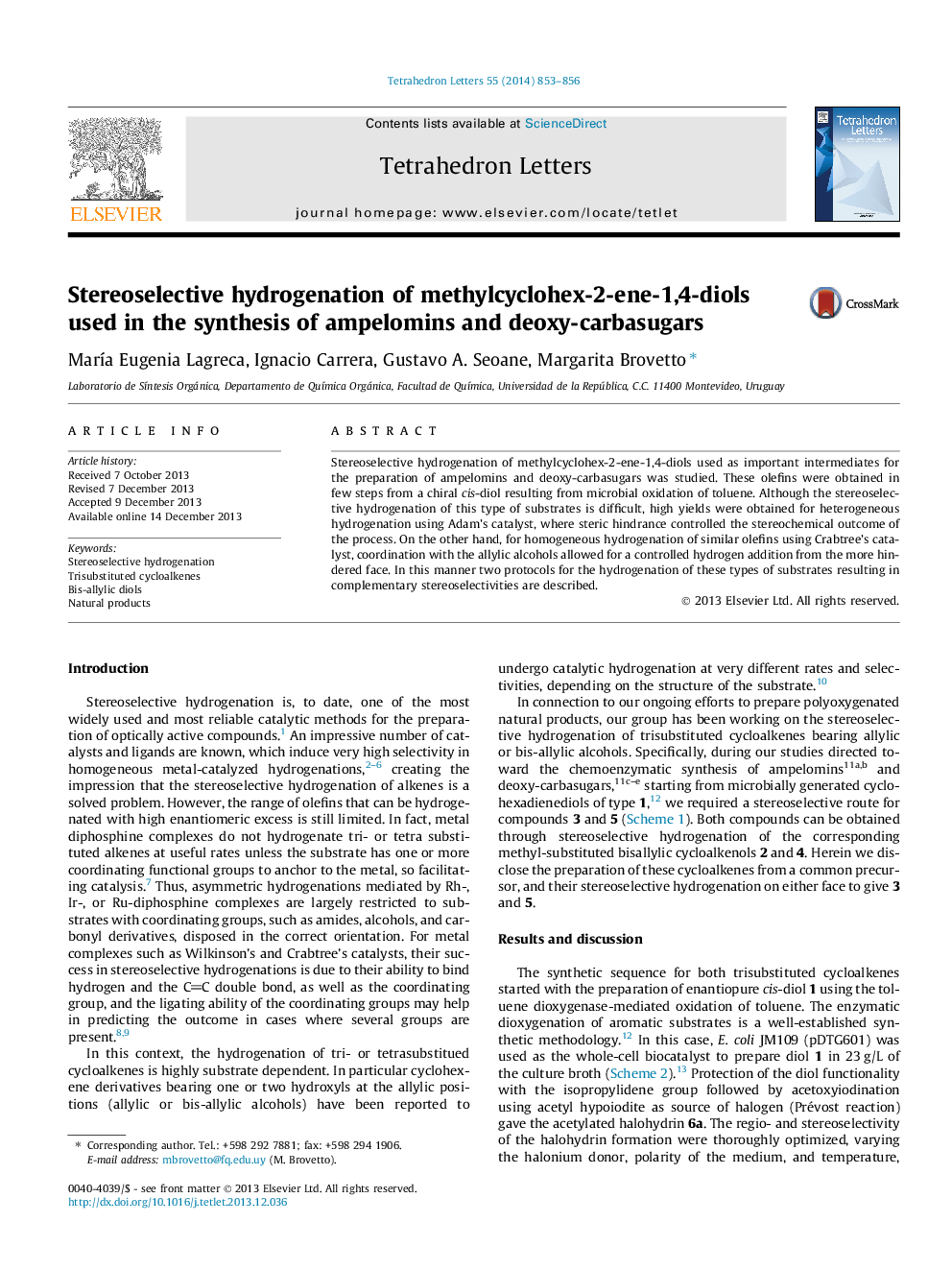
Stereoselective hydrogenation of methylcyclohex-2-ene-1,4-diols used in the synthesis of ampelomins and deoxy-carbasugars
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
موضوعات مرتبط
مهندسی و علوم پایه
شیمی
شیمی آلی
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
Stereoselective hydrogenation of methylcyclohex-2-ene-1,4-diols used as important intermediates for the preparation of ampelomins and deoxy-carbasugars was studied. These olefins were obtained in few steps from a chiral cis-diol resulting from microbial oxidation of toluene. Although the stereoselective hydrogenation of this type of substrates is difficult, high yields were obtained for heterogeneous hydrogenation using Adam's catalyst, where steric hindrance controlled the stereochemical outcome of the process. On the other hand, for homogeneous hydrogenation of similar olefins using Crabtree's catalyst, coordination with the allylic alcohols allowed for a controlled hydrogen addition from the more hindered face. In this manner two protocols for the hydrogenation of these types of substrates resulting in complementary stereoselectivities are described.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Tetrahedron Letters - Volume 55, Issue 4, 22 January 2014, Pages 853-856
Journal: Tetrahedron Letters - Volume 55, Issue 4, 22 January 2014, Pages 853-856
نویسندگان
MarÃa Eugenia Lagreca, Ignacio Carrera, Gustavo A. Seoane, Margarita Brovetto,