| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5276537 | 1385559 | 2007 | 4 صفحه PDF | دانلود رایگان |
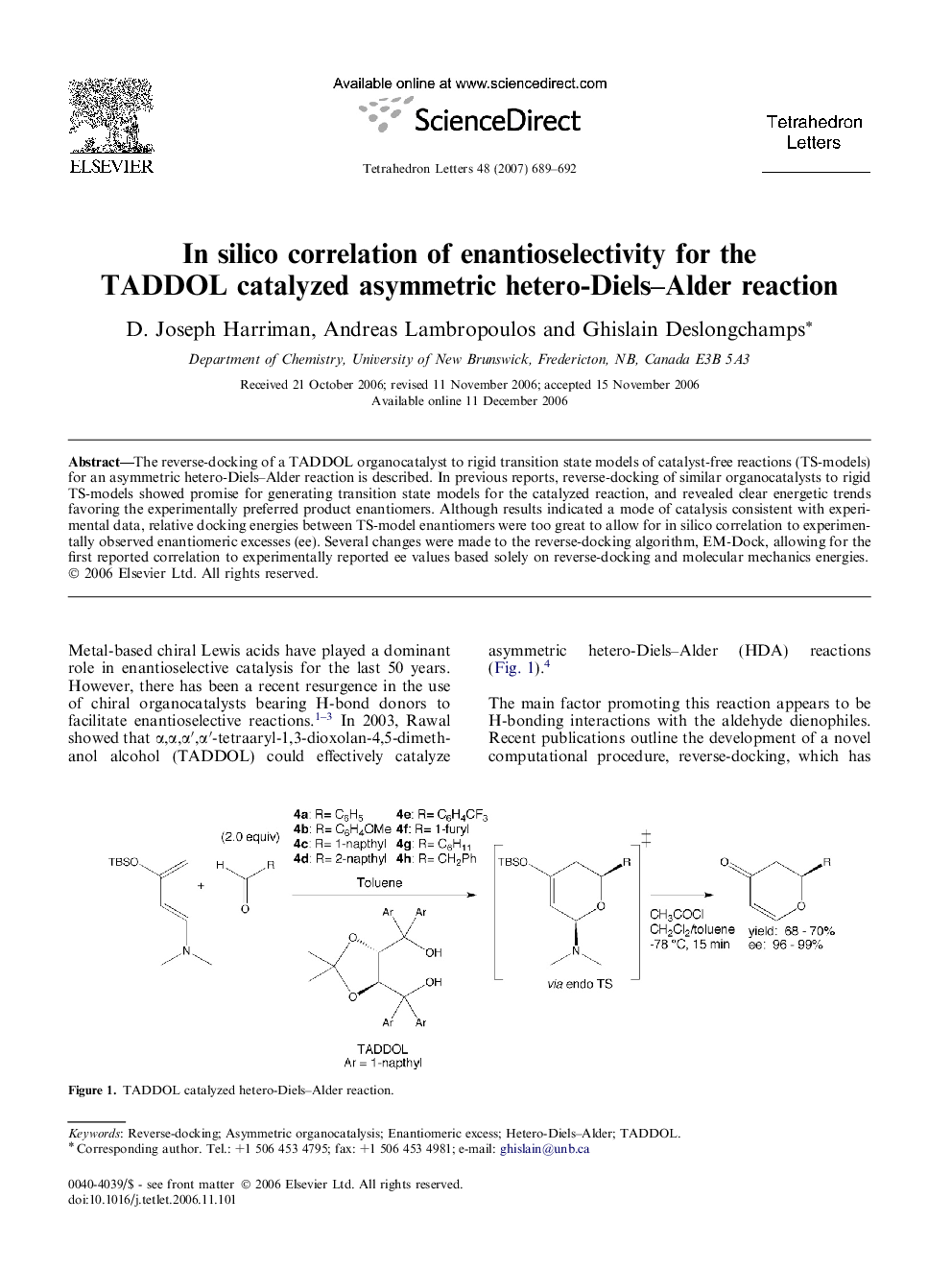
The reverse-docking of a TADDOL organocatalyst to rigid transition state models of catalyst-free reactions (TS-models) for an asymmetric hetero-Diels-Alder reaction is described. In previous reports, reverse-docking of similar organocatalysts to rigid TS-models showed promise for generating transition state models for the catalyzed reaction, and revealed clear energetic trends favoring the experimentally preferred product enantiomers. Although results indicated a mode of catalysis consistent with experimental data, relative docking energies between TS-model enantiomers were too great to allow for in silico correlation to experimentally observed enantiomeric excesses (ee). Several changes were made to the reverse-docking algorithm, EM-Dock, allowing for the first reported correlation to experimentally reported ee values based solely on reverse-docking and molecular mechanics energies.
Journal: Tetrahedron Letters - Volume 48, Issue 4, 22 January 2007, Pages 689-692