| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5278623 | 1385607 | 2006 | 5 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
1,3-Dipolar cycloaddition reactions on carbohydrate-based templates: synthesis of spiro-isoxazolines and 1,2,4-oxadiazoles as glycogen phosphorylase inhibitors
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
کلمات کلیدی
موضوعات مرتبط
مهندسی و علوم پایه
شیمی
شیمی آلی
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
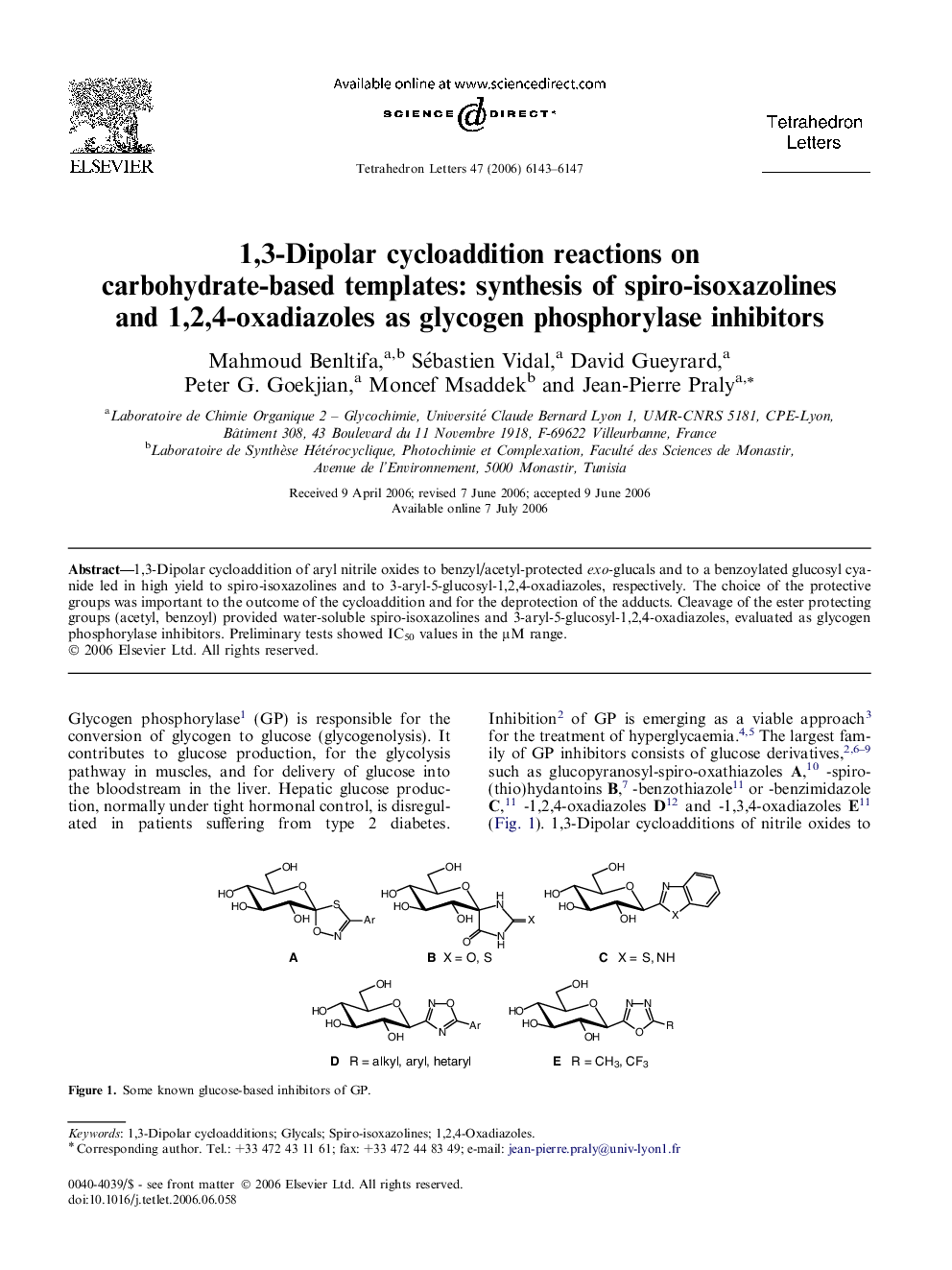
1,3-Dipolar cycloaddition of aryl nitrile oxides to benzyl/acetyl-protected exo-glucals and to a benzoylated glucosyl cyanide led in high yield to spiro-isoxazolines and to 3-aryl-5-glucosyl-1,2,4-oxadiazoles, respectively. The choice of the protective groups was important to the outcome of the cycloaddition and for the deprotection of the adducts. Cleavage of the ester protecting groups (acetyl, benzoyl) provided water-soluble spiro-isoxazolines and 3-aryl-5-glucosyl-1,2,4-oxadiazoles, evaluated as glycogen phosphorylase inhibitors. Preliminary tests showed IC50 values in the μM range.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Tetrahedron Letters - Volume 47, Issue 34, 21 August 2006, Pages 6143-6147
Journal: Tetrahedron Letters - Volume 47, Issue 34, 21 August 2006, Pages 6143-6147
نویسندگان
Mahmoud Benltifa, Sébastien Vidal, David Gueyrard, Peter G. Goekjian, Moncef Msaddek, Jean-Pierre Praly,