| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5370570 | 1503890 | 2017 | 7 صفحه PDF | دانلود رایگان |
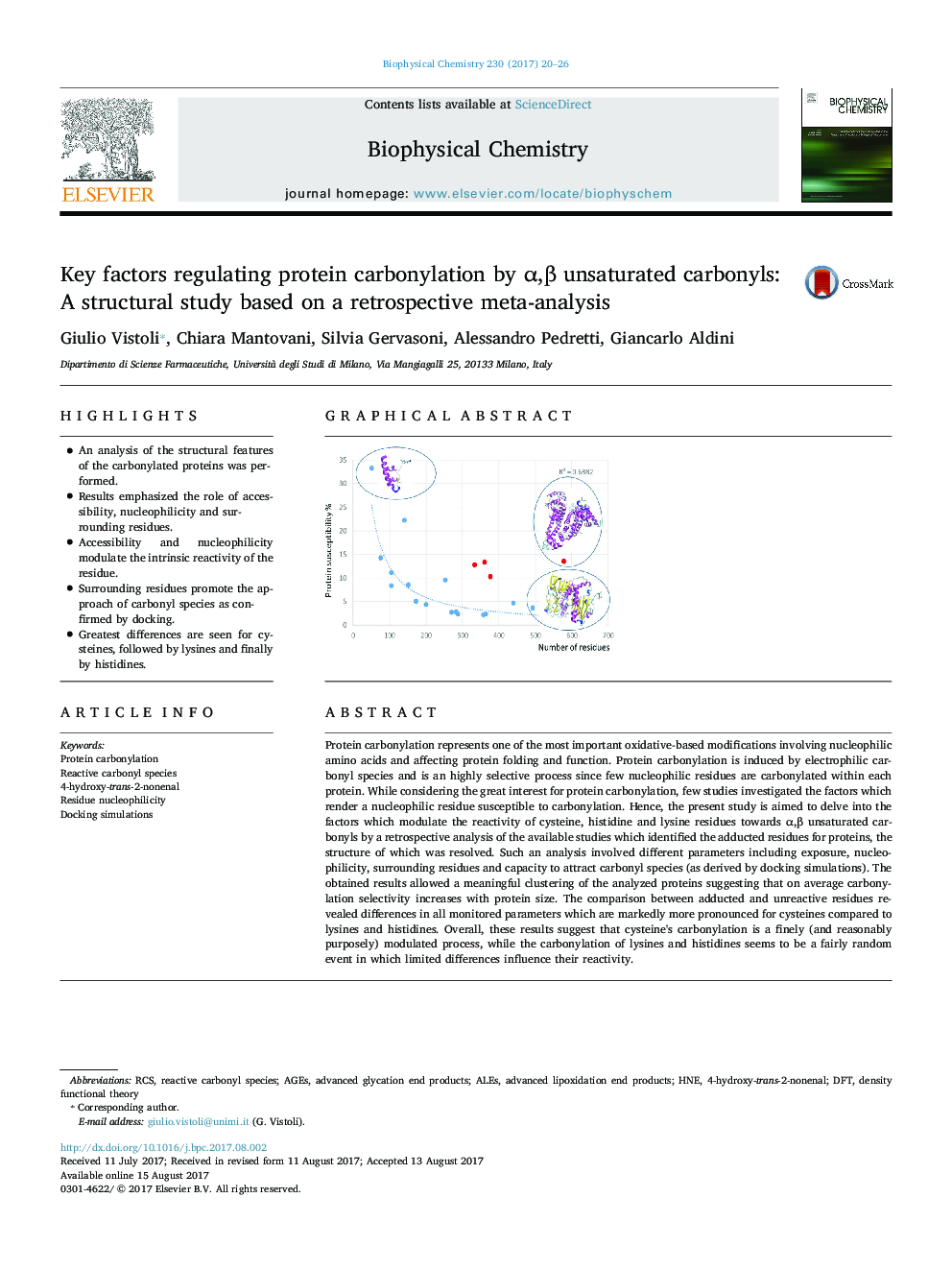
- An analysis of the structural features of the carbonylated proteins was performed.
- Results emphasized the role of accessibility, nucleophilicity and surrounding residues.
- Accessibility and nucleophilicity modulate the intrinsic reactivity of the residue.
- Surrounding residues promote the approach of carbonyl species as confirmed by docking.
- Greatest differences are seen for cysteines, followed by lysines and finally by histidines.
Protein carbonylation represents one of the most important oxidative-based modifications involving nucleophilic amino acids and affecting protein folding and function. Protein carbonylation is induced by electrophilic carbonyl species and is an highly selective process since few nucleophilic residues are carbonylated within each protein. While considering the great interest for protein carbonylation, few studies investigated the factors which render a nucleophilic residue susceptible to carbonylation. Hence, the present study is aimed to delve into the factors which modulate the reactivity of cysteine, histidine and lysine residues towards α,β unsaturated carbonyls by a retrospective analysis of the available studies which identified the adducted residues for proteins, the structure of which was resolved. Such an analysis involved different parameters including exposure, nucleophilicity, surrounding residues and capacity to attract carbonyl species (as derived by docking simulations). The obtained results allowed a meaningful clustering of the analyzed proteins suggesting that on average carbonylation selectivity increases with protein size. The comparison between adducted and unreactive residues revealed differences in all monitored parameters which are markedly more pronounced for cysteines compared to lysines and histidines. Overall, these results suggest that cysteine's carbonylation is a finely (and reasonably purposely) modulated process, while the carbonylation of lysines and histidines seems to be a fairly random event in which limited differences influence their reactivity.
148
Journal: Biophysical Chemistry - Volume 230, November 2017, Pages 20-26