| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5370862 | 1503917 | 2015 | 8 صفحه PDF | دانلود رایگان |
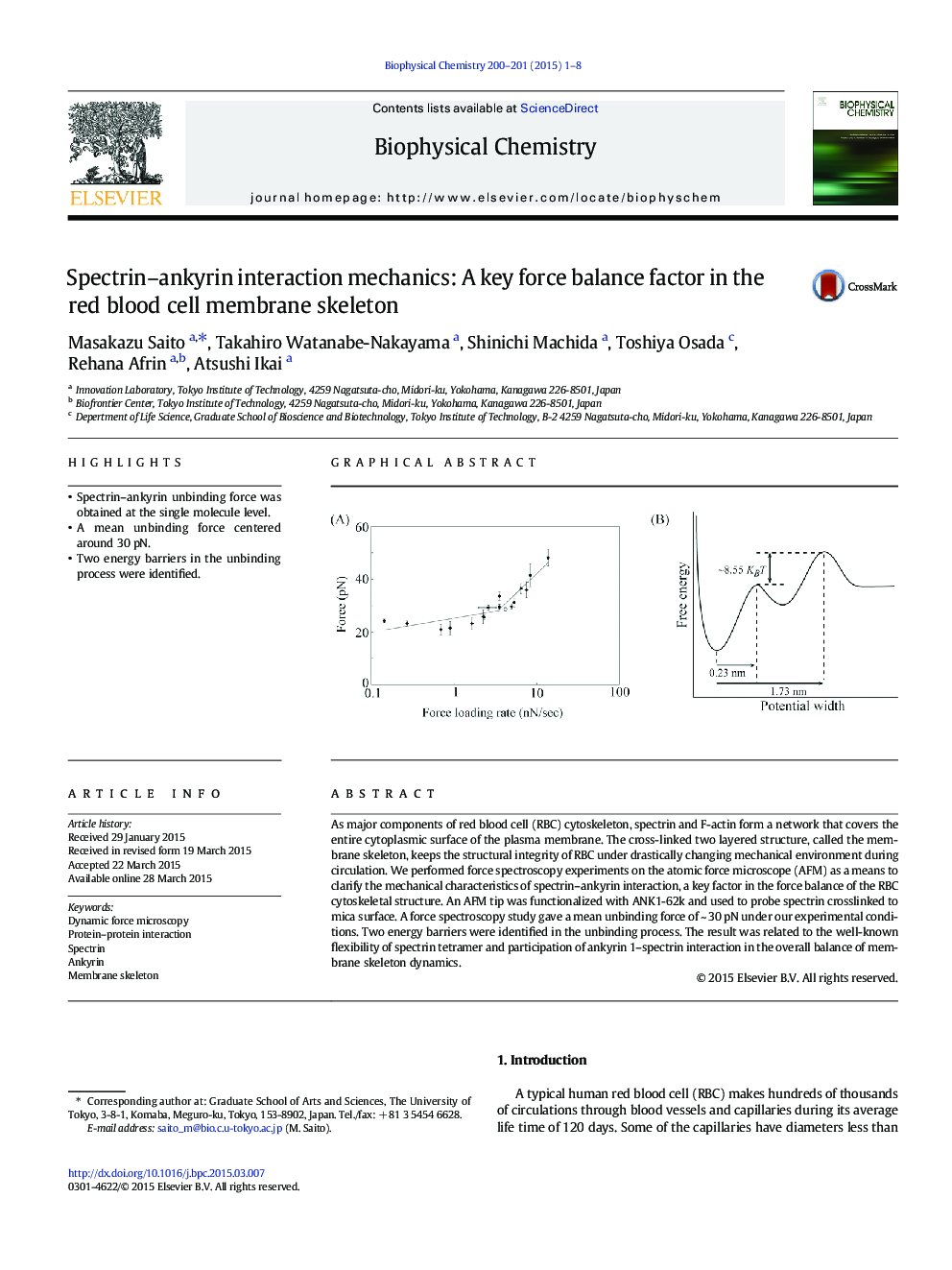
- Spectrin-ankyrin unbinding force was obtained at the single molecule level.
- A mean unbinding force centered around 30Â pN.
- Two energy barriers in the unbinding process were identified.
As major components of red blood cell (RBC) cytoskeleton, spectrin and F-actin form a network that covers the entire cytoplasmic surface of the plasma membrane. The cross-linked two layered structure, called the membrane skeleton, keeps the structural integrity of RBC under drastically changing mechanical environment during circulation. We performed force spectroscopy experiments on the atomic force microscope (AFM) as a means to clarify the mechanical characteristics of spectrin-ankyrin interaction, a key factor in the force balance of the RBC cytoskeletal structure. An AFM tip was functionalized with ANK1-62k and used to probe spectrin crosslinked to mica surface. A force spectroscopy study gave a mean unbinding force of ~Â 30Â pN under our experimental conditions. Two energy barriers were identified in the unbinding process. The result was related to the well-known flexibility of spectrin tetramer and participation of ankyrin 1-spectrin interaction in the overall balance of membrane skeleton dynamics.
Journal: Biophysical Chemistry - Volumes 200â201, MayâJune 2015, Pages 1-8