| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 61596 | 47590 | 2011 | 10 صفحه PDF | دانلود رایگان |
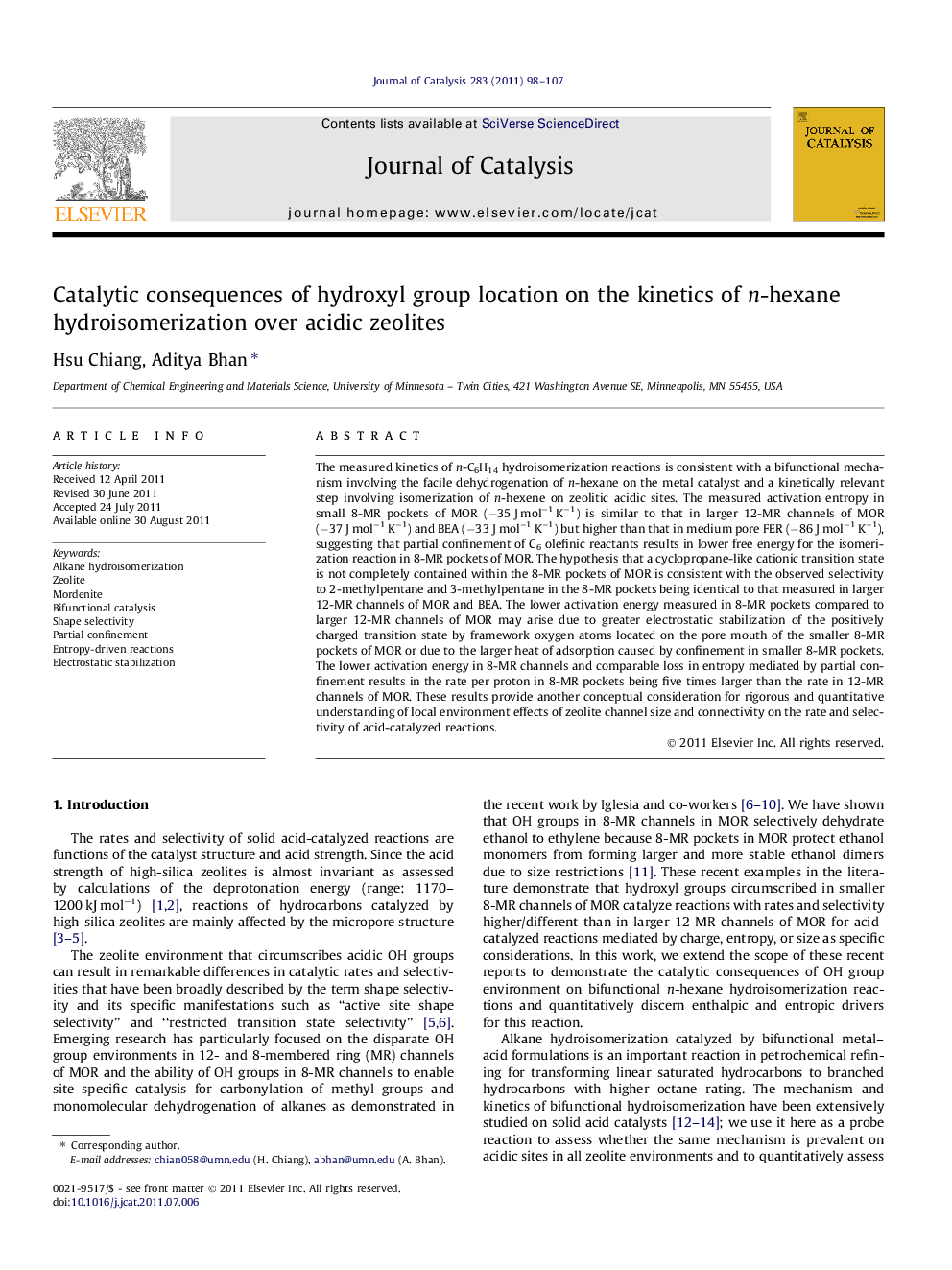
The measured kinetics of n-C6H14 hydroisomerization reactions is consistent with a bifunctional mechanism involving the facile dehydrogenation of n-hexane on the metal catalyst and a kinetically relevant step involving isomerization of n-hexene on zeolitic acidic sites. The measured activation entropy in small 8-MR pockets of MOR (−35 J mol−1 K−1) is similar to that in larger 12-MR channels of MOR (−37 J mol−1 K−1) and BEA (−33 J mol−1 K−1) but higher than that in medium pore FER (−86 J mol−1 K−1), suggesting that partial confinement of C6 olefinic reactants results in lower free energy for the isomerization reaction in 8-MR pockets of MOR. The hypothesis that a cyclopropane-like cationic transition state is not completely contained within the 8-MR pockets of MOR is consistent with the observed selectivity to 2-methylpentane and 3-methylpentane in the 8-MR pockets being identical to that measured in larger 12-MR channels of MOR and BEA. The lower activation energy measured in 8-MR pockets compared to larger 12-MR channels of MOR may arise due to greater electrostatic stabilization of the positively charged transition state by framework oxygen atoms located on the pore mouth of the smaller 8-MR pockets of MOR or due to the larger heat of adsorption caused by confinement in smaller 8-MR pockets. The lower activation energy in 8-MR channels and comparable loss in entropy mediated by partial confinement results in the rate per proton in 8-MR pockets being five times larger than the rate in 12-MR channels of MOR. These results provide another conceptual consideration for rigorous and quantitative understanding of local environment effects of zeolite channel size and connectivity on the rate and selectivity of acid-catalyzed reactions.
The n-hexene molecule is partially confined in the 8-MR side pockets of MOR, which results in entropy of activation and selectivity for isomerization reactions in 8-MR pockets being similar to that in 12-MR channel zeolites; the lower activation energy in 8-MR channels results in catalytic rates per proton being five times higher than those in 12-MR channels of MOR.Figure optionsDownload high-quality image (66 K)Download as PowerPoint slideHighlights
► Rate per proton in different zeolite environments for n-hexane hydroisomerization.
► Selectivity, entropy of activation similar in 8-MR pockets and larger 12-MR channels.
► Higher rates in 8-MR pockets than in 12-MR channels of MOR due to partial confinement.
► Pore size does not predict the occurrence of a particular reaction within zeolites.
Journal: Journal of Catalysis - Volume 283, Issue 1, 6 October 2011, Pages 98–107