| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 64626 | 48363 | 2016 | 6 صفحه PDF | دانلود رایگان |
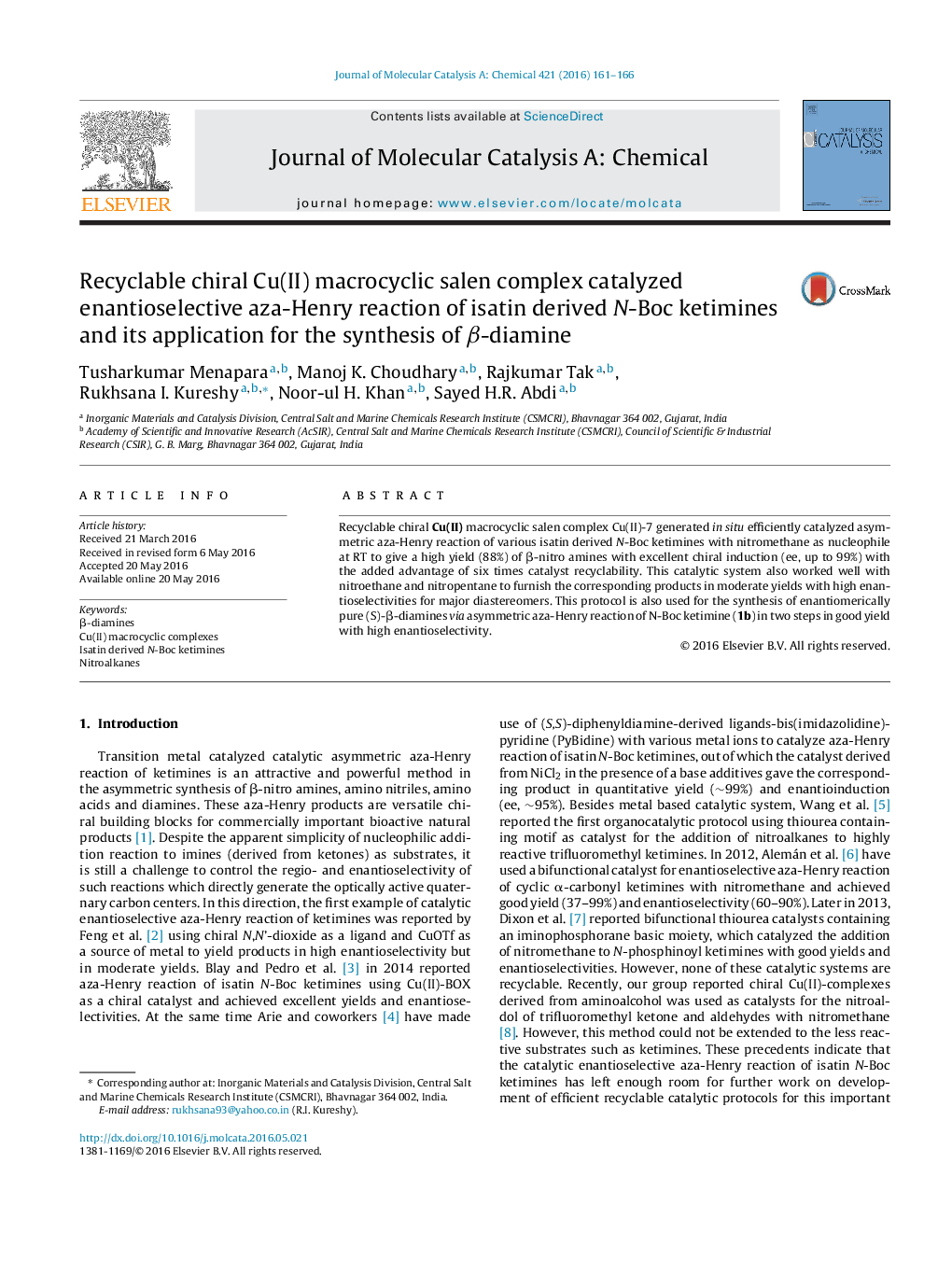
• Chiral Cu(II) macrocyclic salen catalyst with trigol linker was used for the asymmetric aza Henry reaction of various isatin derived N-Boc ketimines.
• Various nitroalkanes were utilized for asymmetric aza Henry reaction with high yield (upto 88%) with ee upto 99% at RT.
• This protocol is used for the synthesis of (S)-β-diamine, precursor for pharmaceutically active molecules in two steps.
• The catalyst worked well up to 6 cycles with retention of catalyst performance.
Recyclable chiral Cu(II) macrocyclic salen complex Cu(II)-7 generated in situ efficiently catalyzed asymmetric aza-Henry reaction of various isatin derived N-Boc ketimines with nitromethane as nucleophile at RT to give a high yield (88%) of β-nitro amines with excellent chiral induction (ee, up to 99%) with the added advantage of six times catalyst recyclability. This catalytic system also worked well with nitroethane and nitropentane to furnish the corresponding products in moderate yields with high enantioselectivities for major diastereomers. This protocol is also used for the synthesis of enantiomerically pure (S)-β-diamines via asymmetric aza-Henry reaction of N-Boc ketimine (1b) in two steps in good yield with high enantioselectivity.
Figure optionsDownload high-quality image (137 K)Download as PowerPoint slide
Journal: Journal of Molecular Catalysis A: Chemical - Volume 421, September 2016, Pages 161–166