| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6465782 | 1422957 | 2017 | 8 صفحه PDF | دانلود رایگان |
- MOFs with acidic/basic groups were synthesized/applied in adsorptive denitrogenation.
- Denitrogenation was improved especially for neutral nitorgen-containing compounds.
- Hydrogen bonding is possible mechanism for the improved adsorptive removal.
- Adsorbents can be hydrogen acceptors for neutral nitorgen-containing compounds.
- Basic nitorgen containing compounds can be adsorbed effectively in low concentration.
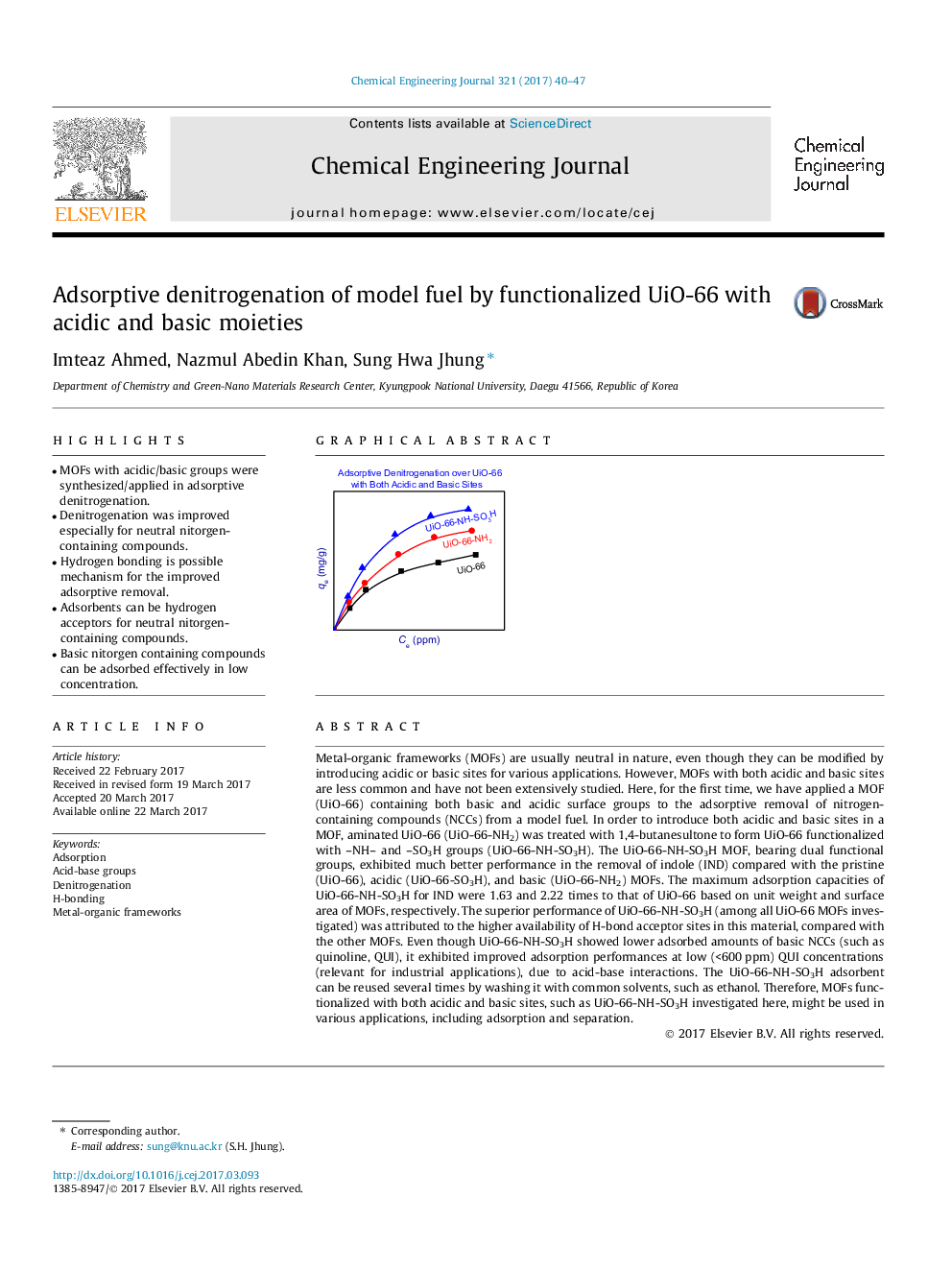
Metal-organic frameworks (MOFs) are usually neutral in nature, even though they can be modified by introducing acidic or basic sites for various applications. However, MOFs with both acidic and basic sites are less common and have not been extensively studied. Here, for the first time, we have applied a MOF (UiO-66) containing both basic and acidic surface groups to the adsorptive removal of nitrogen-containing compounds (NCCs) from a model fuel. In order to introduce both acidic and basic sites in a MOF, aminated UiO-66 (UiO-66-NH2) was treated with 1,4-butanesultone to form UiO-66 functionalized with -NH- and -SO3H groups (UiO-66-NH-SO3H). The UiO-66-NH-SO3H MOF, bearing dual functional groups, exhibited much better performance in the removal of indole (IND) compared with the pristine (UiO-66), acidic (UiO-66-SO3H), and basic (UiO-66-NH2) MOFs. The maximum adsorption capacities of UiO-66-NH-SO3H for IND were 1.63 and 2.22 times to that of UiO-66 based on unit weight and surface area of MOFs, respectively. The superior performance of UiO-66-NH-SO3H (among all UiO-66 MOFs investigated) was attributed to the higher availability of H-bond acceptor sites in this material, compared with the other MOFs. Even though UiO-66-NH-SO3H showed lower adsorbed amounts of basic NCCs (such as quinoline, QUI), it exhibited improved adsorption performances at low (<600Â ppm) QUI concentrations (relevant for industrial applications), due to acid-base interactions. The UiO-66-NH-SO3H adsorbent can be reused several times by washing it with common solvents, such as ethanol. Therefore, MOFs functionalized with both acidic and basic sites, such as UiO-66-NH-SO3H investigated here, might be used in various applications, including adsorption and separation.
129
Journal: Chemical Engineering Journal - Volume 321, 1 August 2017, Pages 40-47