| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6466309 | 1422963 | 2017 | 10 صفحه PDF | دانلود رایگان |
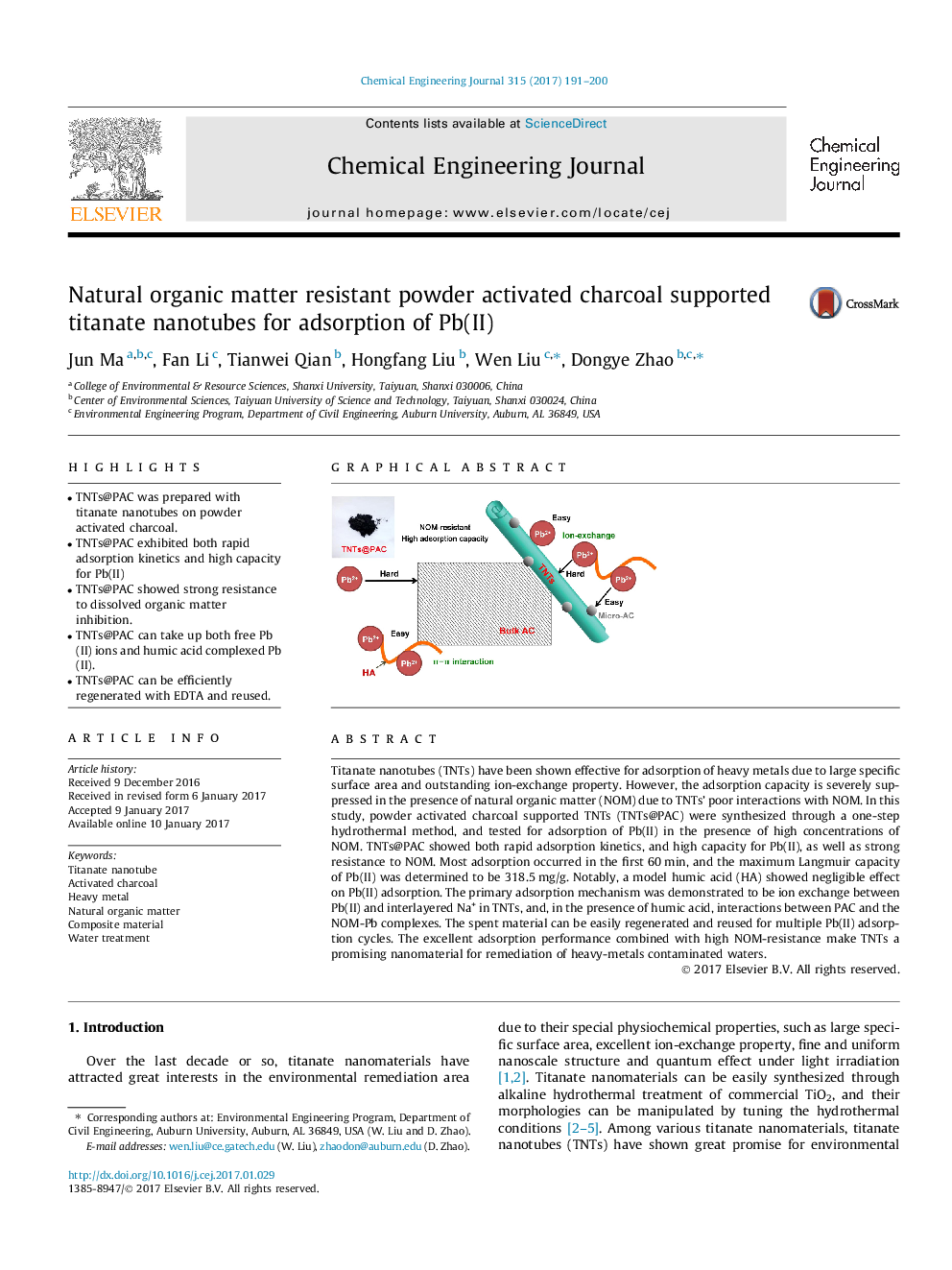
- TNTs@PAC was prepared with titanate nanotubes on powder activated charcoal.
- TNTs@PAC exhibited both rapid adsorption kinetics and high capacity for Pb(II)
- TNTs@PAC showed strong resistance to dissolved organic matter inhibition.
- TNTs@PAC can take up both free Pb(II) ions and humic acid complexed Pb(II).
- TNTs@PAC can be efficiently regenerated with EDTA and reused.
Titanate nanotubes (TNTs) have been shown effective for adsorption of heavy metals due to large specific surface area and outstanding ion-exchange property. However, the adsorption capacity is severely suppressed in the presence of natural organic matter (NOM) due to TNTs' poor interactions with NOM. In this study, powder activated charcoal supported TNTs (TNTs@PAC) were synthesized through a one-step hydrothermal method, and tested for adsorption of Pb(II) in the presence of high concentrations of NOM. TNTs@PAC showed both rapid adsorption kinetics, and high capacity for Pb(II), as well as strong resistance to NOM. Most adsorption occurred in the first 60Â min, and the maximum Langmuir capacity of Pb(II) was determined to be 318.5Â mg/g. Notably, a model humic acid (HA) showed negligible effect on Pb(II) adsorption. The primary adsorption mechanism was demonstrated to be ion exchange between Pb(II) and interlayered Na+ in TNTs, and, in the presence of humic acid, interactions between PAC and the NOM-Pb complexes. The spent material can be easily regenerated and reused for multiple Pb(II) adsorption cycles. The excellent adsorption performance combined with high NOM-resistance make TNTs a promising nanomaterial for remediation of heavy-metals contaminated waters.
112
Journal: Chemical Engineering Journal - Volume 315, 1 May 2017, Pages 191-200