| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6466579 | 1422965 | 2017 | 8 صفحه PDF | دانلود رایگان |
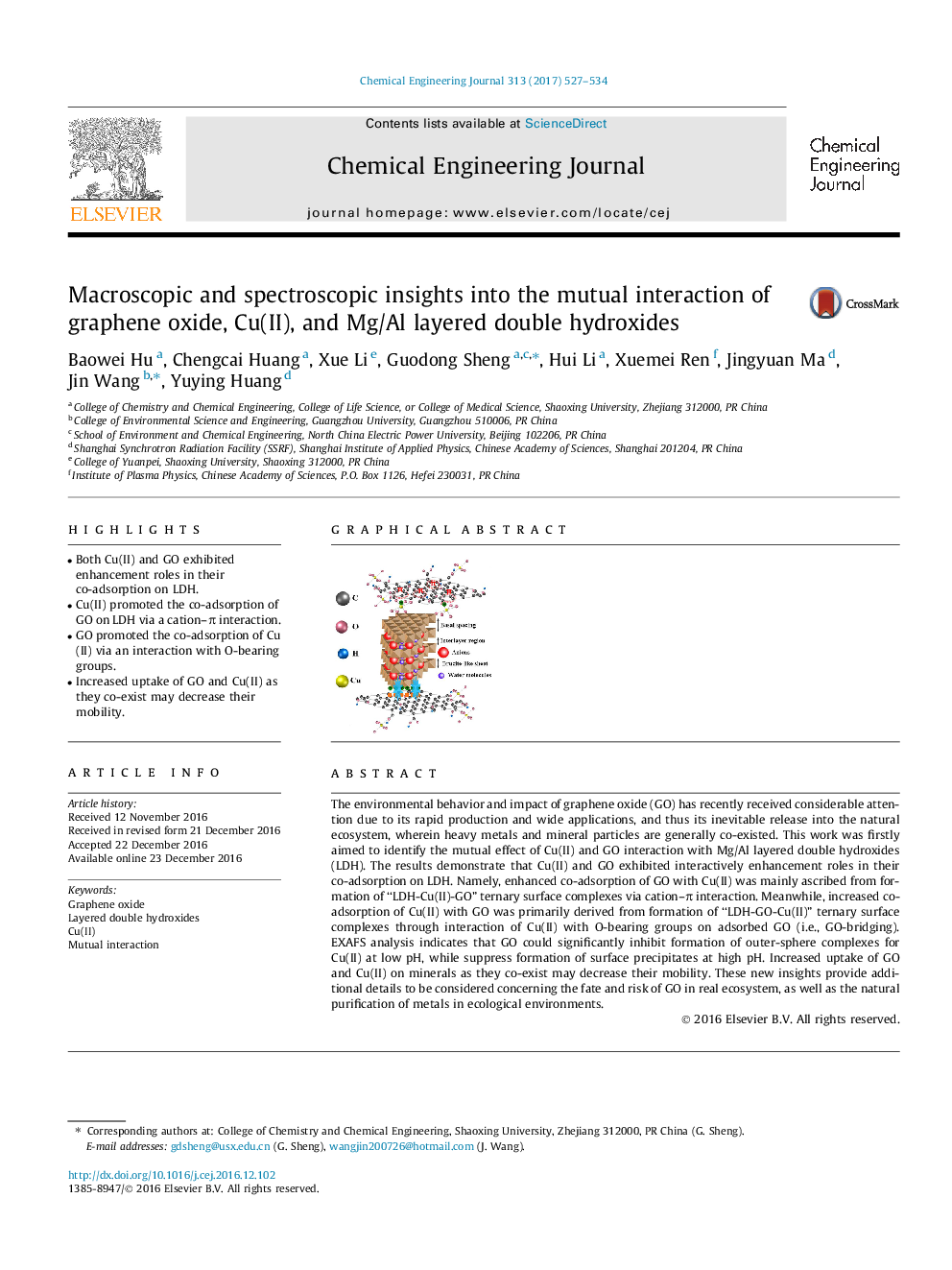
- Both Cu(II) and GO exhibited enhancement roles in their co-adsorption on LDH.
- Cu(II) promoted the co-adsorption of GO on LDH via a cation-Ï interaction.
- GO promoted the co-adsorption of Cu(II) via an interaction with O-bearing groups.
- Increased uptake of GO and Cu(II) as they co-exist may decrease their mobility.
The environmental behavior and impact of graphene oxide (GO) has recently received considerable attention due to its rapid production and wide applications, and thus its inevitable release into the natural ecosystem, wherein heavy metals and mineral particles are generally co-existed. This work was firstly aimed to identify the mutual effect of Cu(II) and GO interaction with Mg/Al layered double hydroxides (LDH). The results demonstrate that Cu(II) and GO exhibited interactively enhancement roles in their co-adsorption on LDH. Namely, enhanced co-adsorption of GO with Cu(II) was mainly ascribed from formation of “LDH-Cu(II)-GO” ternary surface complexes via cation-Ï interaction. Meanwhile, increased co-adsorption of Cu(II) with GO was primarily derived from formation of “LDH-GO-Cu(II)” ternary surface complexes through interaction of Cu(II) with O-bearing groups on adsorbed GO (i.e., GO-bridging). EXAFS analysis indicates that GO could significantly inhibit formation of outer-sphere complexes for Cu(II) at low pH, while suppress formation of surface precipitates at high pH. Increased uptake of GO and Cu(II) on minerals as they co-exist may decrease their mobility. These new insights provide additional details to be considered concerning the fate and risk of GO in real ecosystem, as well as the natural purification of metals in ecological environments.
87
Journal: Chemical Engineering Journal - Volume 313, 1 April 2017, Pages 527-534