| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6466749 | 1422968 | 2017 | 9 صفحه PDF | دانلود رایگان |
• Tailored synthesis of mesoporous TiO2 was achieved using different glycine amounts.
• XRD can be indexed to the anatase and brookite phases for heterojunction TiO2.
• A/B-TiO2 exhibits higher photoactivity than pure TiO2 phases.
• A/B (61.8/38.2%) TiO2 shows 100% CYN degradation under UV–Vis irradiation.
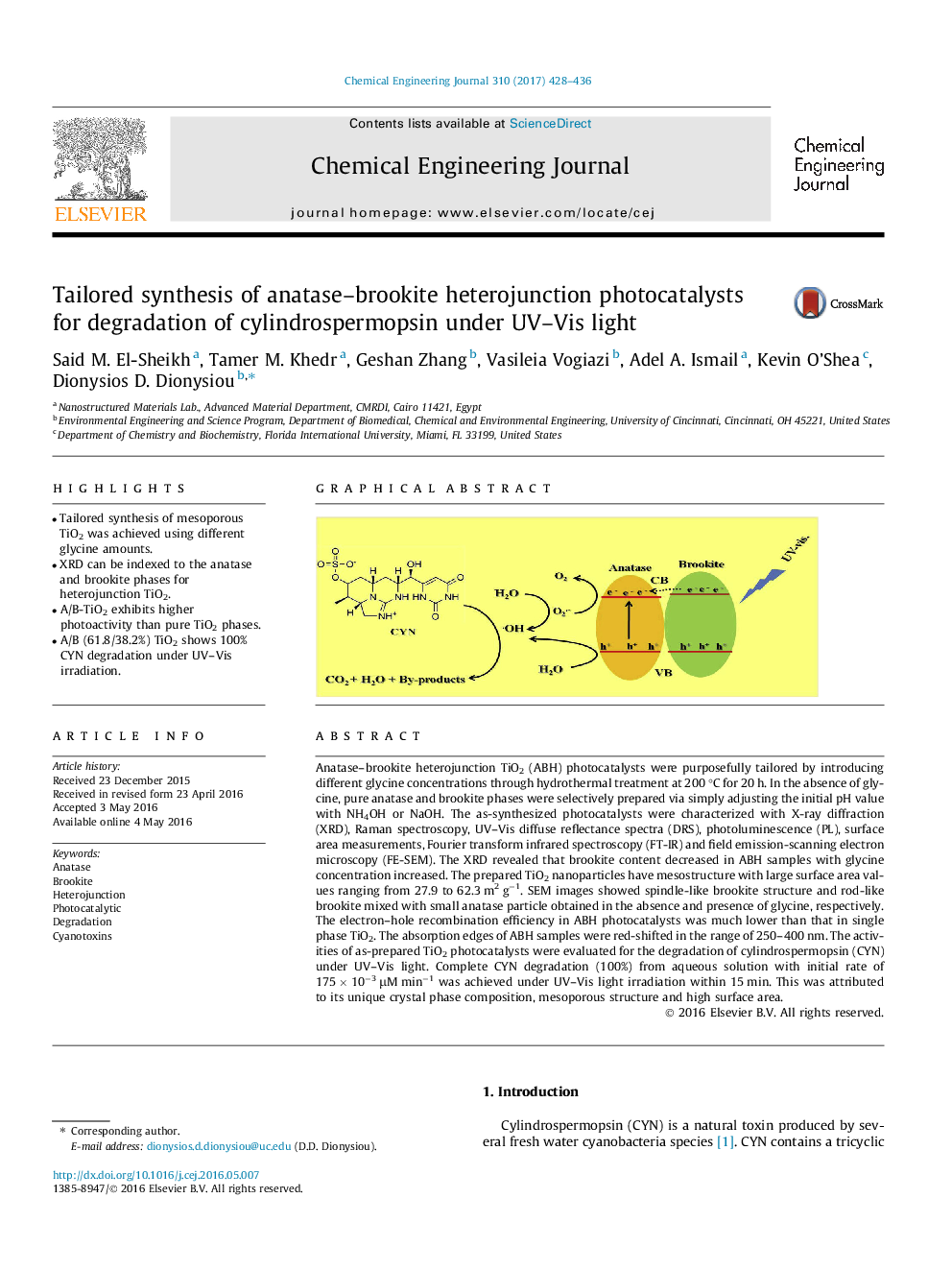
Anatase–brookite heterojunction TiO2 (ABH) photocatalysts were purposefully tailored by introducing different glycine concentrations through hydrothermal treatment at 200 °C for 20 h. In the absence of glycine, pure anatase and brookite phases were selectively prepared via simply adjusting the initial pH value with NH4OH or NaOH. The as-synthesized photocatalysts were characterized with X-ray diffraction (XRD), Raman spectroscopy, UV–Vis diffuse reflectance spectra (DRS), photoluminescence (PL), surface area measurements, Fourier transform infrared spectroscopy (FT-IR) and field emission-scanning electron microscopy (FE-SEM). The XRD revealed that brookite content decreased in ABH samples with glycine concentration increased. The prepared TiO2 nanoparticles have mesostructure with large surface area values ranging from 27.9 to 62.3 m2 g−1. SEM images showed spindle-like brookite structure and rod-like brookite mixed with small anatase particle obtained in the absence and presence of glycine, respectively. The electron–hole recombination efficiency in ABH photocatalysts was much lower than that in single phase TiO2. The absorption edges of ABH samples were red-shifted in the range of 250–400 nm. The activities of as-prepared TiO2 photocatalysts were evaluated for the degradation of cylindrospermopsin (CYN) under UV–Vis light. Complete CYN degradation (100%) from aqueous solution with initial rate of 175 × 10−3 μM min−1 was achieved under UV–Vis light irradiation within 15 min. This was attributed to its unique crystal phase composition, mesoporous structure and high surface area.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 310, Part 2, 15 February 2017, Pages 428–436