| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6475676 | 1424975 | 2017 | 7 صفحه PDF | دانلود رایگان |
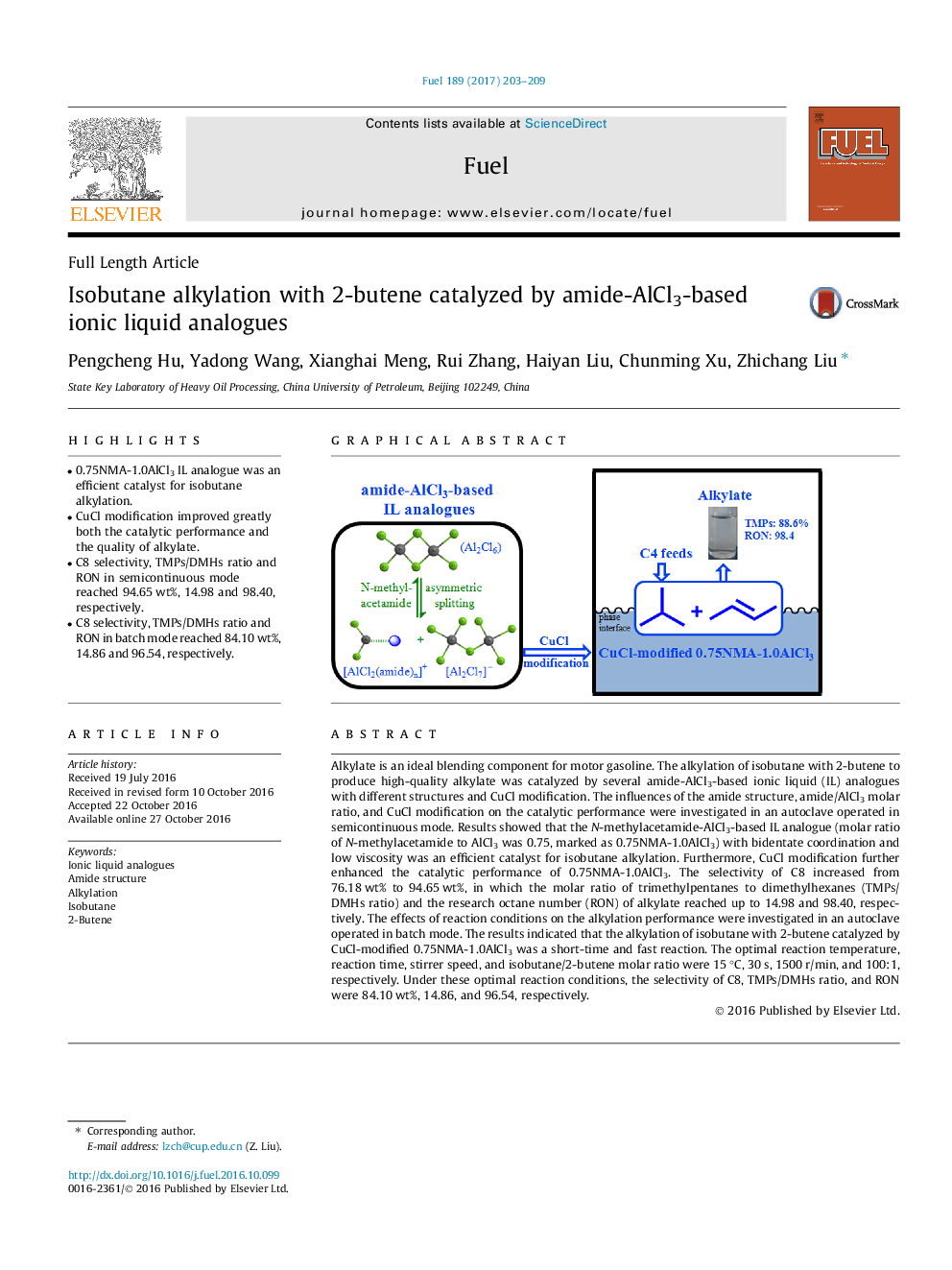
- 0.75NMA-1.0AlCl3 IL analogue was an efficient catalyst for isobutane alkylation.
- CuCl modification improved greatly both the catalytic performance and the quality of alkylate.
- C8 selectivity, TMPs/DMHs ratio and RON in semicontinuous mode reached 94.65Â wt%, 14.98 and 98.40, respectively.
- C8 selectivity, TMPs/DMHs ratio and RON in batch mode reached 84.10Â wt%, 14.86 and 96.54, respectively.
Alkylate is an ideal blending component for motor gasoline. The alkylation of isobutane with 2-butene to produce high-quality alkylate was catalyzed by several amide-AlCl3-based ionic liquid (IL) analogues with different structures and CuCl modification. The influences of the amide structure, amide/AlCl3 molar ratio, and CuCl modification on the catalytic performance were investigated in an autoclave operated in semicontinuous mode. Results showed that the N-methylacetamide-AlCl3-based IL analogue (molar ratio of N-methylacetamide to AlCl3 was 0.75, marked as 0.75NMA-1.0AlCl3) with bidentate coordination and low viscosity was an efficient catalyst for isobutane alkylation. Furthermore, CuCl modification further enhanced the catalytic performance of 0.75NMA-1.0AlCl3. The selectivity of C8 increased from 76.18 wt% to 94.65 wt%, in which the molar ratio of trimethylpentanes to dimethylhexanes (TMPs/DMHs ratio) and the research octane number (RON) of alkylate reached up to 14.98 and 98.40, respectively. The effects of reaction conditions on the alkylation performance were investigated in an autoclave operated in batch mode. The results indicated that the alkylation of isobutane with 2-butene catalyzed by CuCl-modified 0.75NMA-1.0AlCl3 was a short-time and fast reaction. The optimal reaction temperature, reaction time, stirrer speed, and isobutane/2-butene molar ratio were 15 °C, 30 s, 1500 r/min, and 100:1, respectively. Under these optimal reaction conditions, the selectivity of C8, TMPs/DMHs ratio, and RON were 84.10 wt%, 14.86, and 96.54, respectively.
98
Journal: Fuel - Volume 189, 1 February 2017, Pages 203-209