| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 64811 | 48371 | 2016 | 11 صفحه PDF | دانلود رایگان |
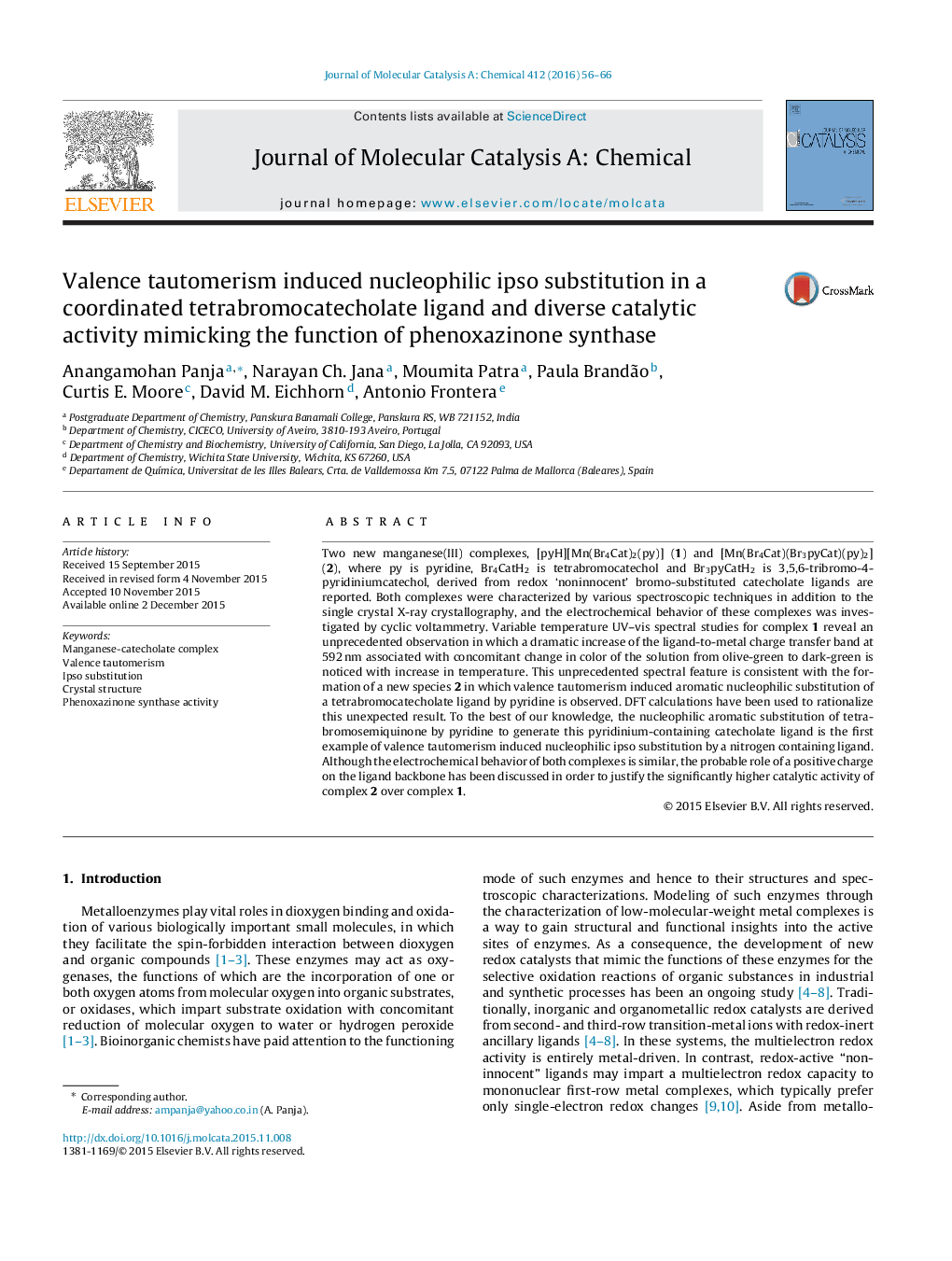
• Manganese(III) complex with redox-noninnocent tetrabromocatecholate ligand.
• Valence tautomerism induced aromatic nucleophilic substitution of a tetrabromocatecholate ligand by pyridine, supported by DFT calculations.
• The first example of valence tautomerism induced nucleophilic ipso substitution by a nitrogen containing ligand.
• Diverse catalytic activity mimicking the function of phenoxazinone synthase.
Two new manganese(III) complexes, [pyH][Mn(Br4Cat)2(py)] (1) and [Mn(Br4Cat)(Br3pyCat)(py)2] (2), where py is pyridine, Br4CatH2 is tetrabromocatechol and Br3pyCatH2 is 3,5,6-tribromo-4-pyridiniumcatechol, derived from redox ‘noninnocent’ bromo-substituted catecholate ligands are reported. Both complexes were characterized by various spectroscopic techniques in addition to the single crystal X-ray crystallography, and the electrochemical behavior of these complexes was investigated by cyclic voltammetry. Variable temperature UV–vis spectral studies for complex 1 reveal an unprecedented observation in which a dramatic increase of the ligand-to-metal charge transfer band at 592 nm associated with concomitant change in color of the solution from olive-green to dark-green is noticed with increase in temperature. This unprecedented spectral feature is consistent with the formation of a new species 2 in which valence tautomerism induced aromatic nucleophilic substitution of a tetrabromocatecholate ligand by pyridine is observed. DFT calculations have been used to rationalize this unexpected result. To the best of our knowledge, the nucleophilic aromatic substitution of tetrabromosemiquinone by pyridine to generate this pyridinium-containing catecholate ligand is the first example of valence tautomerism induced nucleophilic ipso substitution by a nitrogen containing ligand. Although the electrochemical behavior of both complexes is similar, the probable role of a positive charge on the ligand backbone has been discussed in order to justify the significantly higher catalytic activity of complex 2 over complex 1.
The nucleophilic aromatic substitution of tetrabromosemiquinone by pyridine to generate a pyridinium-containing catecholate ligand is the first example of valence tautomerism induced nucleophilic ipso substitution by a nitrogen containing ligand. The probable role of a positive charge on the ligand backbone on diverse catalytic activity has been explored.Figure optionsDownload high-quality image (142 K)Download as PowerPoint slide
Journal: Journal of Molecular Catalysis A: Chemical - Volume 412, February 2016, Pages 56–66