| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 64812 | 48371 | 2016 | 11 صفحه PDF | دانلود رایگان |
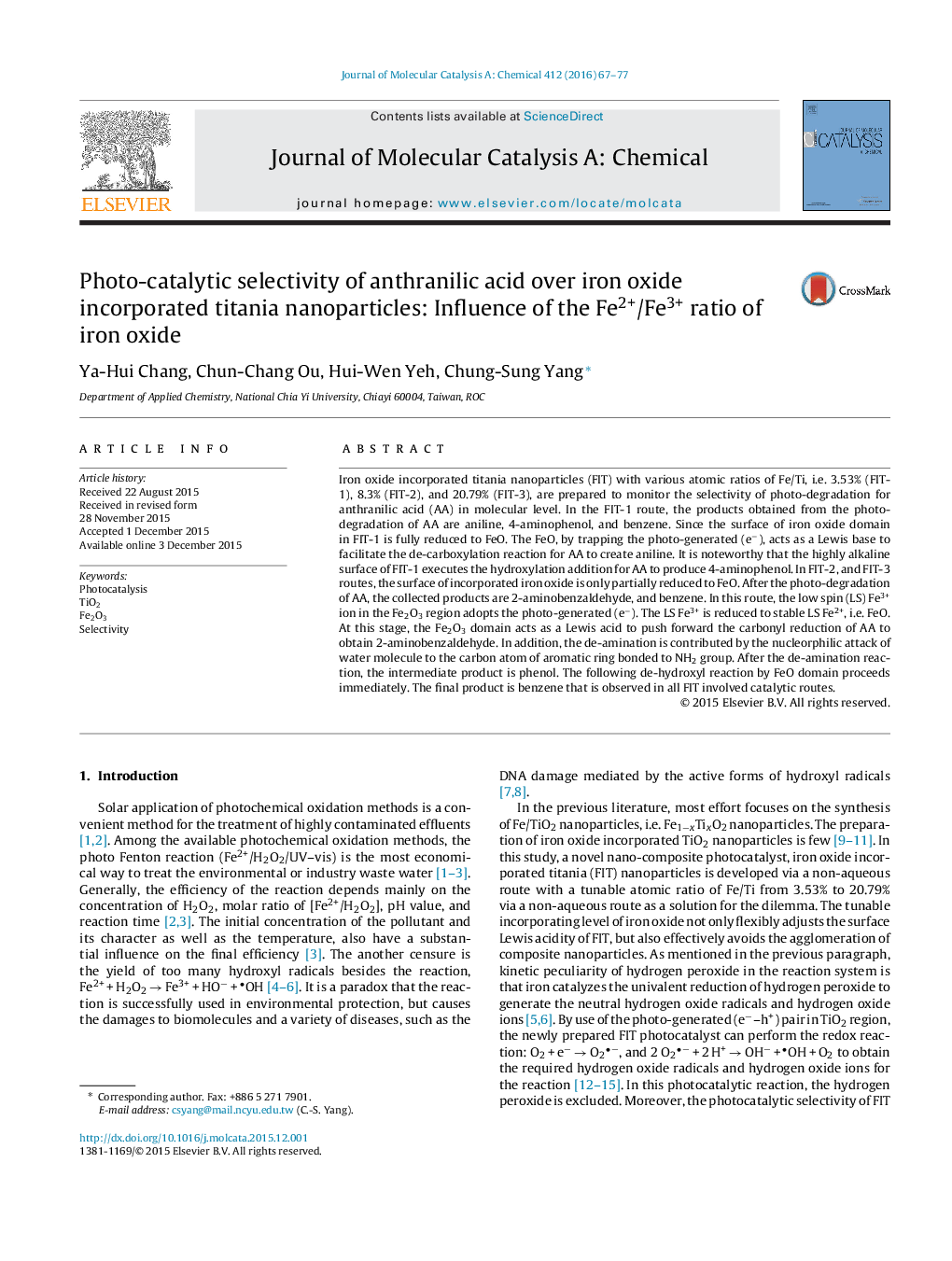
• The ratio of Fe2+/Fe3+ decreases as the ratio of Fe/Ti increases in iron oxide incorporated titania nanoparticles (FIT).
• The high ratio of Fe2+/Fe3+ show the Fe2O3 surface is reduced to FeO completely.
• The moiety of FeO acts as a Lewis base in photocatalysis.
• The low ratio of Fe2+/Fe3+ show the Fe2O3 surface is only partially reduced to FeO.
• The moiety of Fe2O3 acts as a Lewis acid in photocatalysis.
Iron oxide incorporated titania nanoparticles (FIT) with various atomic ratios of Fe/Ti, i.e. 3.53% (FIT-1), 8.3% (FIT-2), and 20.79% (FIT-3), are prepared to monitor the selectivity of photo-degradation for anthranilic acid (AA) in molecular level. In the FIT-1 route, the products obtained from the photo-degradation of AA are aniline, 4-aminophenol, and benzene. Since the surface of iron oxide domain in FIT-1 is fully reduced to FeO. The FeO, by trapping the photo-generated (e−), acts as a Lewis base to facilitate the de-carboxylation reaction for AA to create aniline. It is noteworthy that the highly alkaline surface of FIT-1 executes the hydroxylation addition for AA to produce 4-aminophenol. In FIT-2, and FIT-3 routes, the surface of incorporated iron oxide is only partially reduced to FeO. After the photo-degradation of AA, the collected products are 2-aminobenzaldehyde, and benzene. In this route, the low spin (LS) Fe3+ ion in the Fe2O3 region adopts the photo-generated (e−). The LS Fe3+ is reduced to stable LS Fe2+, i.e. FeO. At this stage, the Fe2O3 domain acts as a Lewis acid to push forward the carbonyl reduction of AA to obtain 2-aminobenzaldehyde. In addition, the de-amination is contributed by the nucleorphilic attack of water molecule to the carbon atom of aromatic ring bonded to NH2 group. After the de-amination reaction, the intermediate product is phenol. The following de-hydroxyl reaction by FeO domain proceeds immediately. The final product is benzene that is observed in all FIT involved catalytic routes.
The selectivity of photo-degradation is tunable via the ratio of Fe2+/Fe3+ in FIT. In the route of high ratio of Fe2+/Fe3+ (FIT-1), the de-carboxylation and hydroxylation addition reaction prevail. The observed product is aniline and 4-aminophenol. In the low ratio of Fe2+/Fe3+ (FIT-2, FIT-3), the carbonyl reduction reaction is dominate. The product collected from the reaction is 2-aminobenzaldehyde.Figure optionsDownload high-quality image (124 K)Download as PowerPoint slide
Journal: Journal of Molecular Catalysis A: Chemical - Volume 412, February 2016, Pages 67–77