| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 64939 | 48375 | 2015 | 8 صفحه PDF | دانلود رایگان |
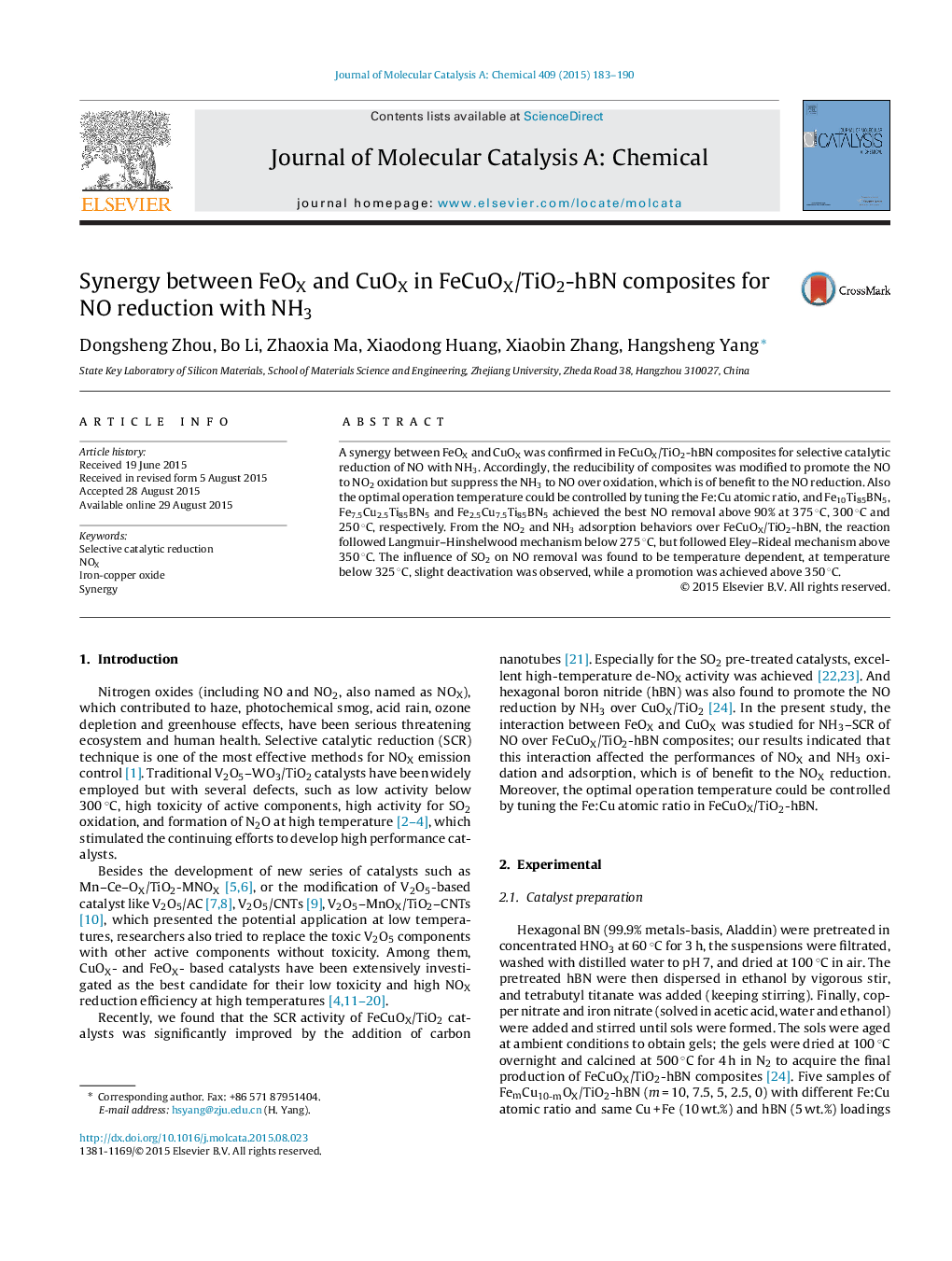
• A synergy between FeOX and CuOX was confirmed in FeCuOX/TiO2-hBN.
• The optimal De-NOX temperature could be engineered.
• The De-NOX reaction followed Langmuir–Hinshelwood mechanism below 275 °C.
• The De-NOX reaction followed Eley–Rideal mechanism above 350 °C.
A synergy between FeOX and CuOX was confirmed in FeCuOX/TiO2-hBN composites for selective catalytic reduction of NO with NH3. Accordingly, the reducibility of composites was modified to promote the NO to NO2 oxidation but suppress the NH3 to NO over oxidation, which is of benefit to the NO reduction. Also the optimal operation temperature could be controlled by tuning the Fe:Cu atomic ratio, and Fe10Ti85BN5, Fe7.5Cu2.5Ti85BN5 and Fe2.5Cu7.5Ti85BN5 achieved the best NO removal above 90% at 375 °C, 300 °C and 250 °C, respectively. From the NO2 and NH3 adsorption behaviors over FeCuOX/TiO2-hBN, the reaction followed Langmuir–Hinshelwood mechanism below 275 °C, but followed Eley–Rideal mechanism above 350 °C. The influence of SO2 on NO removal was found to be temperature dependent, at temperature below 325 °C, slight deactivation was observed, while a promotion was achieved above 350 °C.
Figure optionsDownload high-quality image (230 K)Download as PowerPoint slide
Journal: Journal of Molecular Catalysis A: Chemical - Volume 409, 1 December 2015, Pages 183–190