| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 67800 | 48495 | 2007 | 10 صفحه PDF | دانلود رایگان |
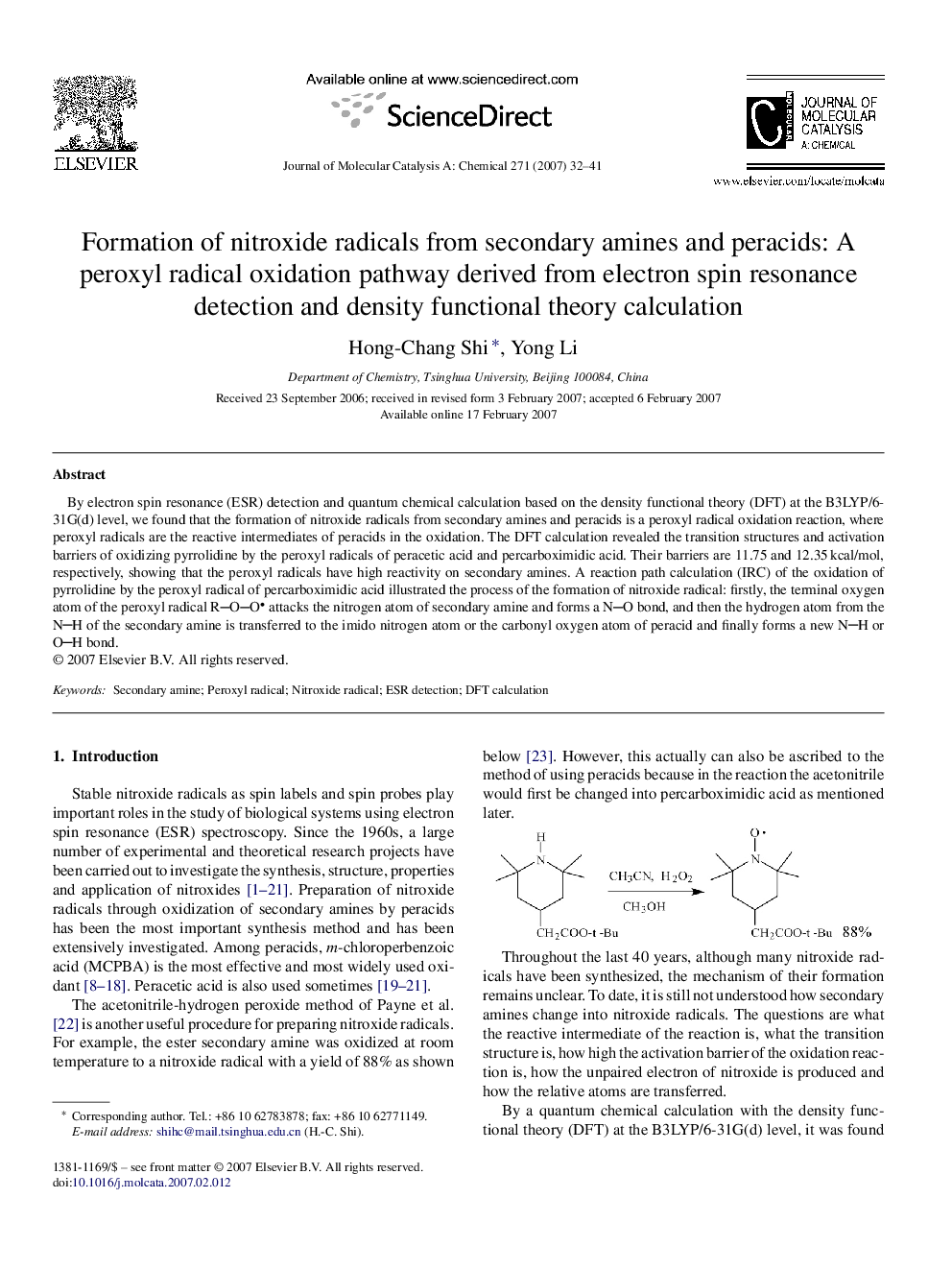
By electron spin resonance (ESR) detection and quantum chemical calculation based on the density functional theory (DFT) at the B3LYP/6-31G(d) level, we found that the formation of nitroxide radicals from secondary amines and peracids is a peroxyl radical oxidation reaction, where peroxyl radicals are the reactive intermediates of peracids in the oxidation. The DFT calculation revealed the transition structures and activation barriers of oxidizing pyrrolidine by the peroxyl radicals of peracetic acid and percarboximidic acid. Their barriers are 11.75 and 12.35 kcal/mol, respectively, showing that the peroxyl radicals have high reactivity on secondary amines. A reaction path calculation (IRC) of the oxidation of pyrrolidine by the peroxyl radical of percarboximidic acid illustrated the process of the formation of nitroxide radical: firstly, the terminal oxygen atom of the peroxyl radical ROO attacks the nitrogen atom of secondary amine and forms a NO bond, and then the hydrogen atom from the NH of the secondary amine is transferred to the imido nitrogen atom or the carbonyl oxygen atom of peracid and finally forms a new NH or OH bond.
Formation of nitroxide radicals from secondary amines and peracids is a peroxyl radical oxidation reaction with low activation barriers. By a calculation with DFT method at B3LYP/6-31G(d) level, it was found that the activation barriers of oxidizing pyrrolidine by the peroxyl radicals of peracetic acid and percarboximidic acid are 11.75 and 12.35 kcal/mol, respectively. Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Catalysis A: Chemical - Volume 271, Issues 1–2, 18 June 2007, Pages 32–41