| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 679803 | 1459958 | 2015 | 9 صفحه PDF | دانلود رایگان |
• Functionalized cells are obtained by a simple and environmentally friendly route.
• This new adsorbent has an intracellular CaCO3 mineral scaffold.
• The CaCO3 mineral scaffold could promote the uptake of the heavy metal ions.
• The adsorption capacity of functionalized cells is more increased than yeast cell.
• Functionalized cells could be a promising material for removal of heavy metals.
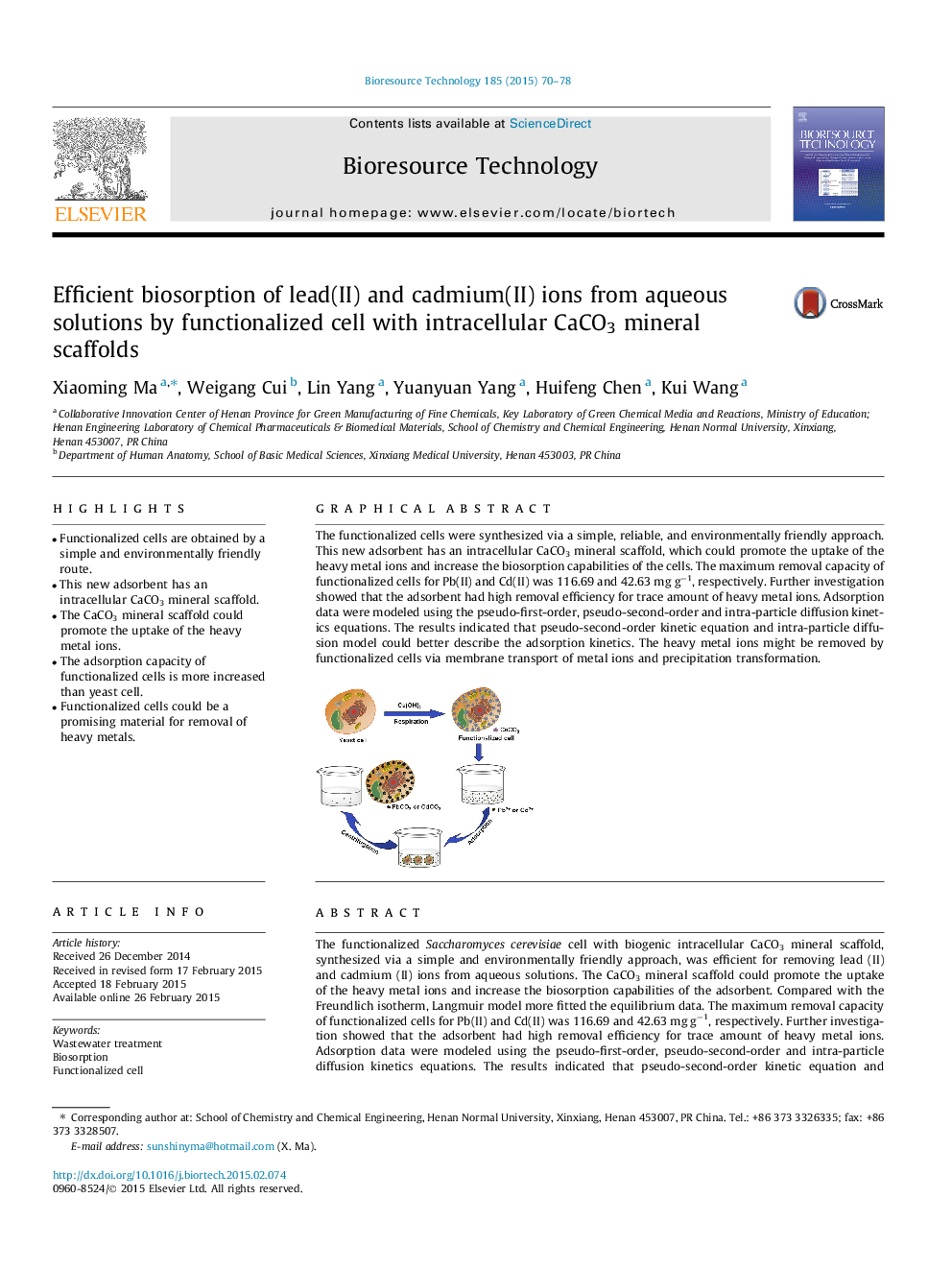
The functionalized Saccharomyces cerevisiae cell with biogenic intracellular CaCO3 mineral scaffold, synthesized via a simple and environmentally friendly approach, was efficient for removing lead (II) and cadmium (II) ions from aqueous solutions. The CaCO3 mineral scaffold could promote the uptake of the heavy metal ions and increase the biosorption capabilities of the adsorbent. Compared with the Freundlich isotherm, Langmuir model more fitted the equilibrium data. The maximum removal capacity of functionalized cells for Pb(II) and Cd(II) was 116.69 and 42.63 mg g−1, respectively. Further investigation showed that the adsorbent had high removal efficiency for trace amount of heavy metal ions. Adsorption data were modeled using the pseudo-first-order, pseudo-second-order and intra-particle diffusion kinetics equations. The results indicated that pseudo-second-order kinetic equation and intra-particle diffusion model could better describe the adsorption kinetics. The heavy metal ions might be removed by functionalized cells via membrane transport of metal ions and precipitation transformation.
The functionalized cells were synthesized via a simple, reliable, and environmentally friendly approach. This new adsorbent has an intracellular CaCO3 mineral scaffold, which could promote the uptake of the heavy metal ions and increase the biosorption capabilities of the cells. The maximum removal capacity of functionalized cells for Pb(II) and Cd(II) was 116.69 and 42.63 mg g−1, respectively. Further investigation showed that the adsorbent had high removal efficiency for trace amount of heavy metal ions. Adsorption data were modeled using the pseudo-first-order, pseudo-second-order and intra-particle diffusion kinetics equations. The results indicated that pseudo-second-order kinetic equation and intra-particle diffusion model could better describe the adsorption kinetics. The heavy metal ions might be removed by functionalized cells via membrane transport of metal ions and precipitation transformation.Figure optionsDownload as PowerPoint slide
Journal: Bioresource Technology - Volume 185, June 2015, Pages 70–78