| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 10162369 | 1114327 | 2014 | 9 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
Orally Administered DTPA Penta-Ethyl Ester for the Decorporation of Inhaled 241Am
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
کلمات کلیدی
موضوعات مرتبط
علوم پزشکی و سلامت
داروسازی، سم شناسی و علوم دارویی
اکتشاف دارویی
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
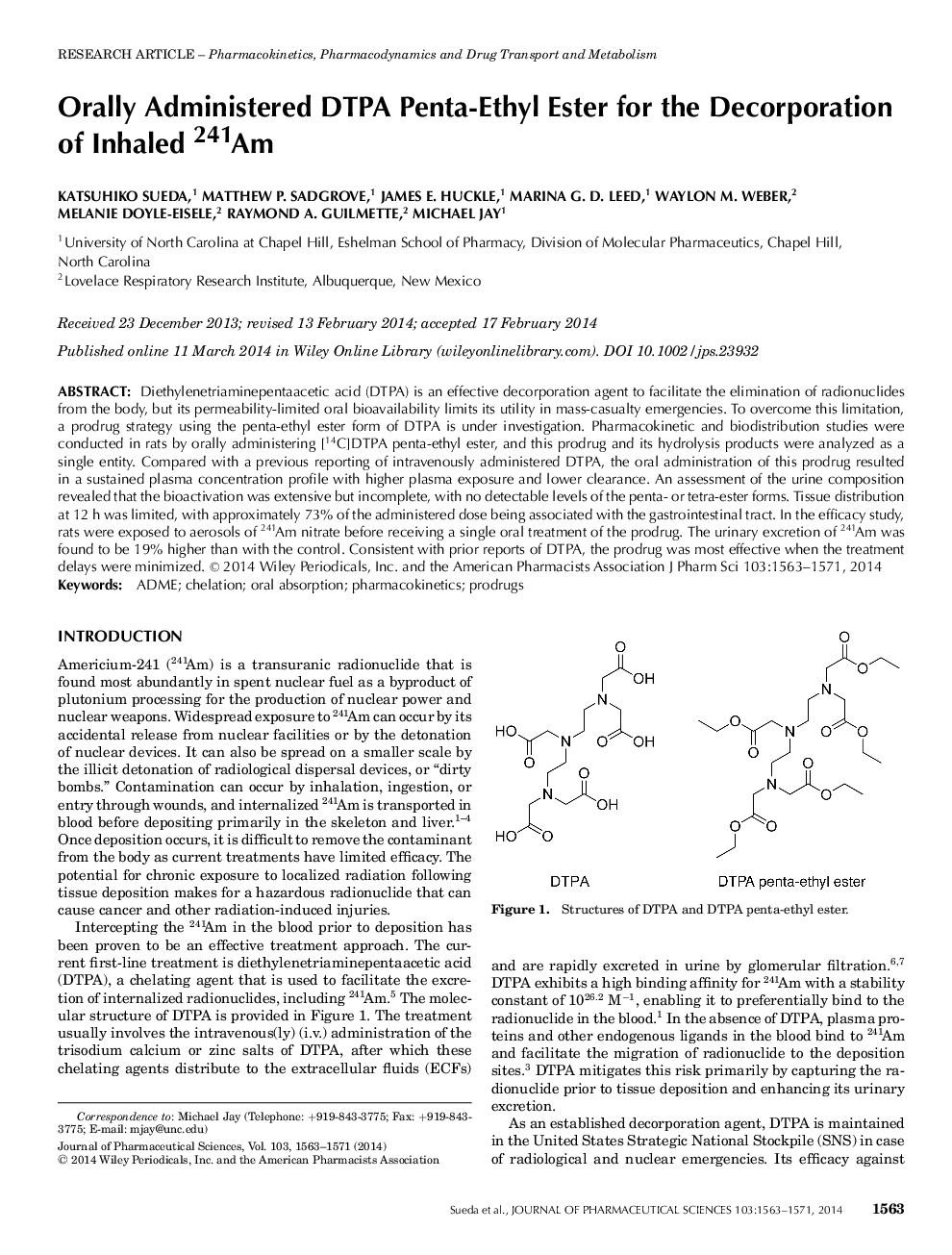
Diethylenetriaminepentaacetic acid (DTPA) is an effective decorporation agent to facilitate the elimination of radionuclides from the body, but its permeability-limited oral bioavailability limits its utility in mass-casualty emergencies. To overcome this limitation, a prodrug strategy using the penta-ethyl ester form of DTPA is under investigation. Pharmacokinetic and biodistribution studies were conducted in rats by orally administering [14C]DTPA penta-ethyl ester, and this prodrug and its hydrolysis products were analyzed as a single entity. Compared with a previous reporting of intravenously administered DTPA, the oral administration of this prodrug resulted in a sustained plasma concentration profile with higher plasma exposure and lower clearance. An assessment of the urine composition revealed that the bioactivation was extensive but incomplete, with no detectable levels of the penta- or tetra-ester forms. Tissue distribution at 12Â h was limited, with approximately 73% of the administered dose being associated with the gastrointestinal tract. In the efficacy study, rats were exposed to aerosols of 241Am nitrate before receiving a single oral treatment of the prodrug. The urinary excretion of 241Am was found to be 19% higher than with the control. Consistent with prior reports of DTPA, the prodrug was most effective when the treatment delays were minimized. © 2014 Wiley Periodicals, Inc. and the American Pharmacists Association.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Journal of Pharmaceutical Sciences - Volume 103, Issue 5, May 2014, Pages 1563-1571
Journal: Journal of Pharmaceutical Sciences - Volume 103, Issue 5, May 2014, Pages 1563-1571
نویسندگان
Katsuhiko Sueda, Matthew P. Sadgrove, James E. Huckle, Marina G.D. Leed, Waylon M. Weber, Melanie Doyle-Eisele, Raymond A. Guilmette, Michael Jay,