| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1165547 | 1491065 | 2013 | 7 صفحه PDF | دانلود رایگان |
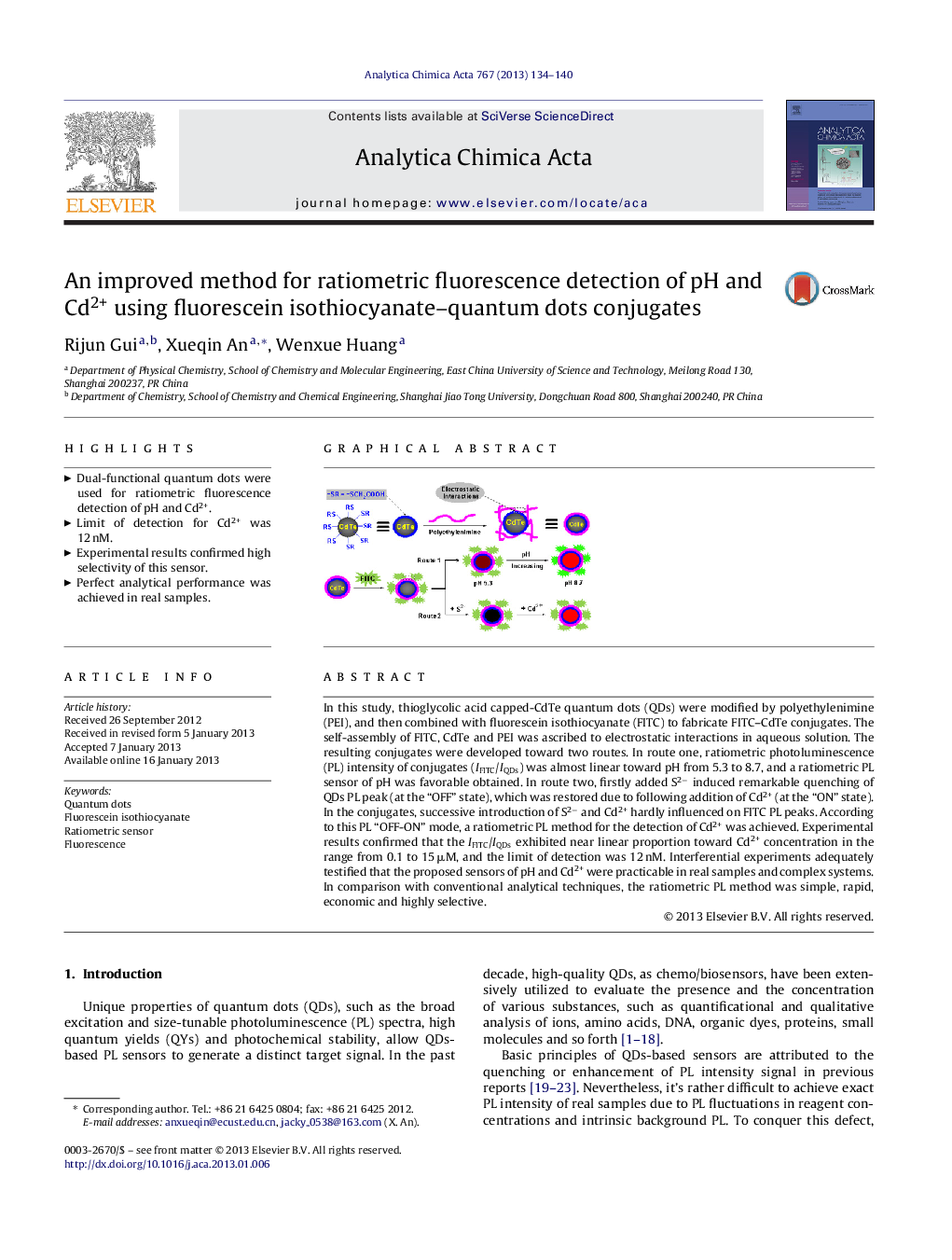
In this study, thioglycolic acid capped-CdTe quantum dots (QDs) were modified by polyethylenimine (PEI), and then combined with fluorescein isothiocyanate (FITC) to fabricate FITC–CdTe conjugates. The self-assembly of FITC, CdTe and PEI was ascribed to electrostatic interactions in aqueous solution. The resulting conjugates were developed toward two routes. In route one, ratiometric photoluminescence (PL) intensity of conjugates (IFITC/IQDs) was almost linear toward pH from 5.3 to 8.7, and a ratiometric PL sensor of pH was favorable obtained. In route two, firstly added S2− induced remarkable quenching of QDs PL peak (at the “OFF” state), which was restored due to following addition of Cd2+ (at the “ON” state). In the conjugates, successive introduction of S2− and Cd2+ hardly influenced on FITC PL peaks. According to this PL “OFF-ON” mode, a ratiometric PL method for the detection of Cd2+ was achieved. Experimental results confirmed that the IFITC/IQDs exhibited near linear proportion toward Cd2+ concentration in the range from 0.1 to 15 μM, and the limit of detection was 12 nM. Interferential experiments adequately testified that the proposed sensors of pH and Cd2+ were practicable in real samples and complex systems. In comparison with conventional analytical techniques, the ratiometric PL method was simple, rapid, economic and highly selective.
Figure optionsDownload as PowerPoint slideHighlights
► Dual-functional quantum dots were used for ratiometric fluorescence detection of pH and Cd2+.
► Limit of detection for Cd2+ was 12 nM.
► Experimental results confirmed high selectivity of this sensor.
► Perfect analytical performance was achieved in real samples.
Journal: Analytica Chimica Acta - Volume 767, 12 March 2013, Pages 134–140