| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1195271 | 964307 | 2009 | 11 صفحه PDF | دانلود رایگان |
In this work, we describe two different methods for generating protonated S-nitrosocysteine in the gas phase. The first method involves a gas-phase reaction of protonated cysteine with t-butylnitrite, while the second method uses a solution-based transnitrosylation reaction of cysteine with S-nitrosoglutathione followed by transfer of the resulting S-nitrosocysteine into the gas phase by electrospray ionization mass spectrometry (ESI-MS). Independent of the way it was formed, protonated S-nitrosocysteine readily fragments via bond homolysis to form a long-lived radical cation of cysteine (Cys
• +), which fragments under collision-induced dissociation (CID) conditions via losses in the following relative abundance order:
• COOH ⪢ CH2S >
• CH2SH ≈ H2S. Deuterium labeling experiments were performed to study the mechanisms leading to these pathways. DFT calculations were also used to probe aspects of the fragmentation of protonated S-nitrosocysteine and the radical cation of cysteine. NO loss is found to be the lowest energy channel for the former ion, while the initially formed distonic Cys
• + with a sulfur radical site undergoes proton and/or H atom transfer reactions that precede the losses of CH2S,
• COOH,
• CH2SH, and H2S.
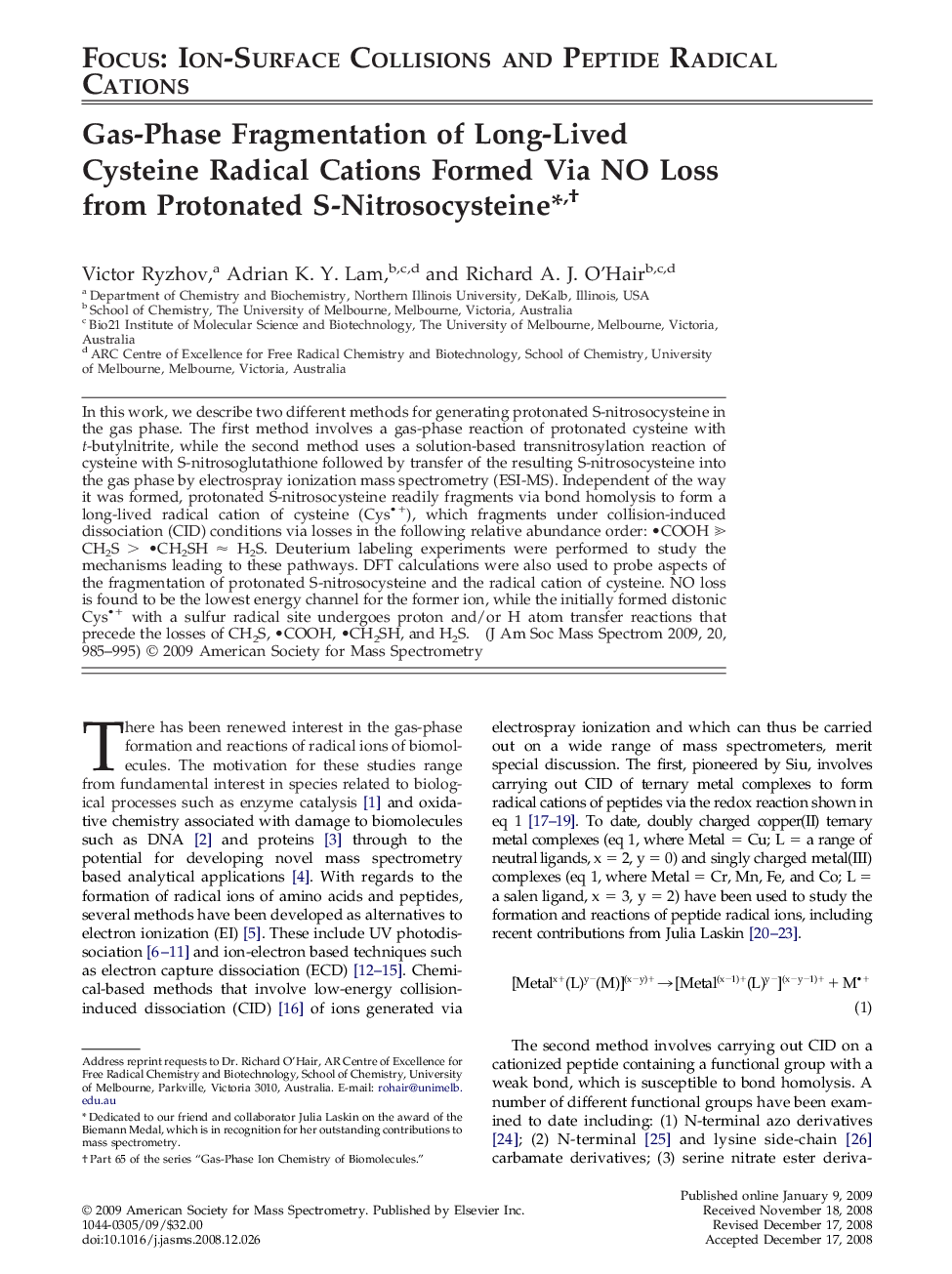
Graphical AbstractCID of protonated S-nitrosocysteine yields the cysteine radical cation (Figure a), which fragments under CID via losses of the following relative abundance order:
• COOH ⪢ CH2S >
• CH2SH ≈ H2S (Figure b).Figure optionsDownload high-quality image (63 K)Download as PowerPoint slide
Journal: Journal of the American Society for Mass Spectrometry - Volume 20, Issue 6, June 2009, Pages 985–995