| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1195272 | 964307 | 2009 | 10 صفحه PDF | دانلود رایگان |
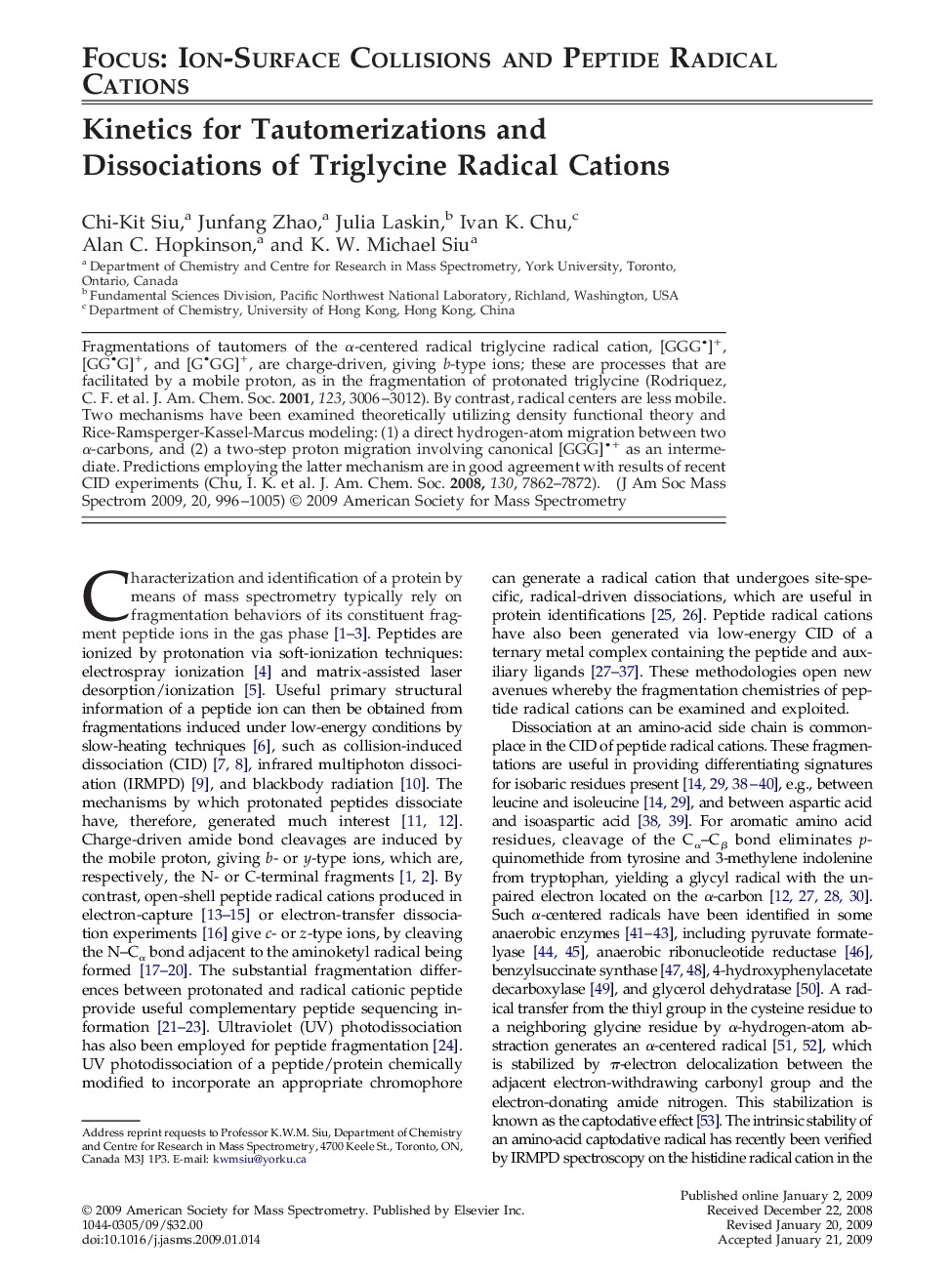
Fragmentations of tautomers of the α-centered radical triglycine radical cation, [GGG
• ]+, [GG
• G]+, and [G
• GG]+, are charge-driven, giving b-type ions; these are processes that are facilitated by a mobile proton, as in the fragmentation of protonated triglycine (Rodriquez, C. F. et al. J. Am. Chem. Soc. 2001, 123, 3006–3012). By contrast, radical centers are less mobile. Two mechanisms have been examined theoretically utilizing density functional theory and Rice-Ramsperger-Kassel-Marcus modeling: (1) a direct hydrogen-atom migration between two α-carbons, and (2) a two-step proton migration involving canonical [GGG]
• + as an intermediate. Predictions employing the latter mechanism are in good agreement with results of recent CID experiments (Chu, I. K. et al. J. Am. Chem. Soc. 2008, 130, 7862–7872).
Graphical AbstractCompared with direct α-hydrogen-atom migration (1), interconversion between [GGG
• ]+ and [G
• GG]+in a two-step proton migration involving a canonical [GGG]
• + intermediate (2) is faster.Figure optionsDownload high-quality image (120 K)Download as PowerPoint slide
Journal: Journal of the American Society for Mass Spectrometry - Volume 20, Issue 6, June 2009, Pages 996–1005