| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230340 | 1495238 | 2014 | 10 صفحه PDF | دانلود رایگان |
• Synthesis of mononuclear Cu(II) hydrazone complexes.
• The Cu(II) complexes structure was elucidated using different characterization techniques.
• Computational studies of the first order kinetic reactions.
• Antimicrobial activities of all isolated compound were studied.
The chelation behaviour of 4-((E)-2-(1-(thiophen-2-yl)ethylidene)hydrazinyl)-1-(4-methoxyphenyl)-1H-pyrrole-3-carbonitrile (HL) towards Cu(II) ions has been investigated. These Cu(II) complexes are characterized by elemental analyses, molar-solid conductance, ESR, FTIR and electronic spectral studies. Also, the kinetic and thermodynamic parameters (Ea, A, ΔH, ΔS, ΔG) for all thermal decomposition steps have been evaluated using Coats–Redfern and Horowitz–Metzger methods. Furthermore, antimicrobial activity of the ligand and its complexes were studied against Gram-negative bacteria: Escherichia coli, Gram-positive Bacillus cereus, Bacillus subtilis and pathogenic fungi Pseudomonas aeruginosa by using minimum inhibitory concentrations (MICs) method.
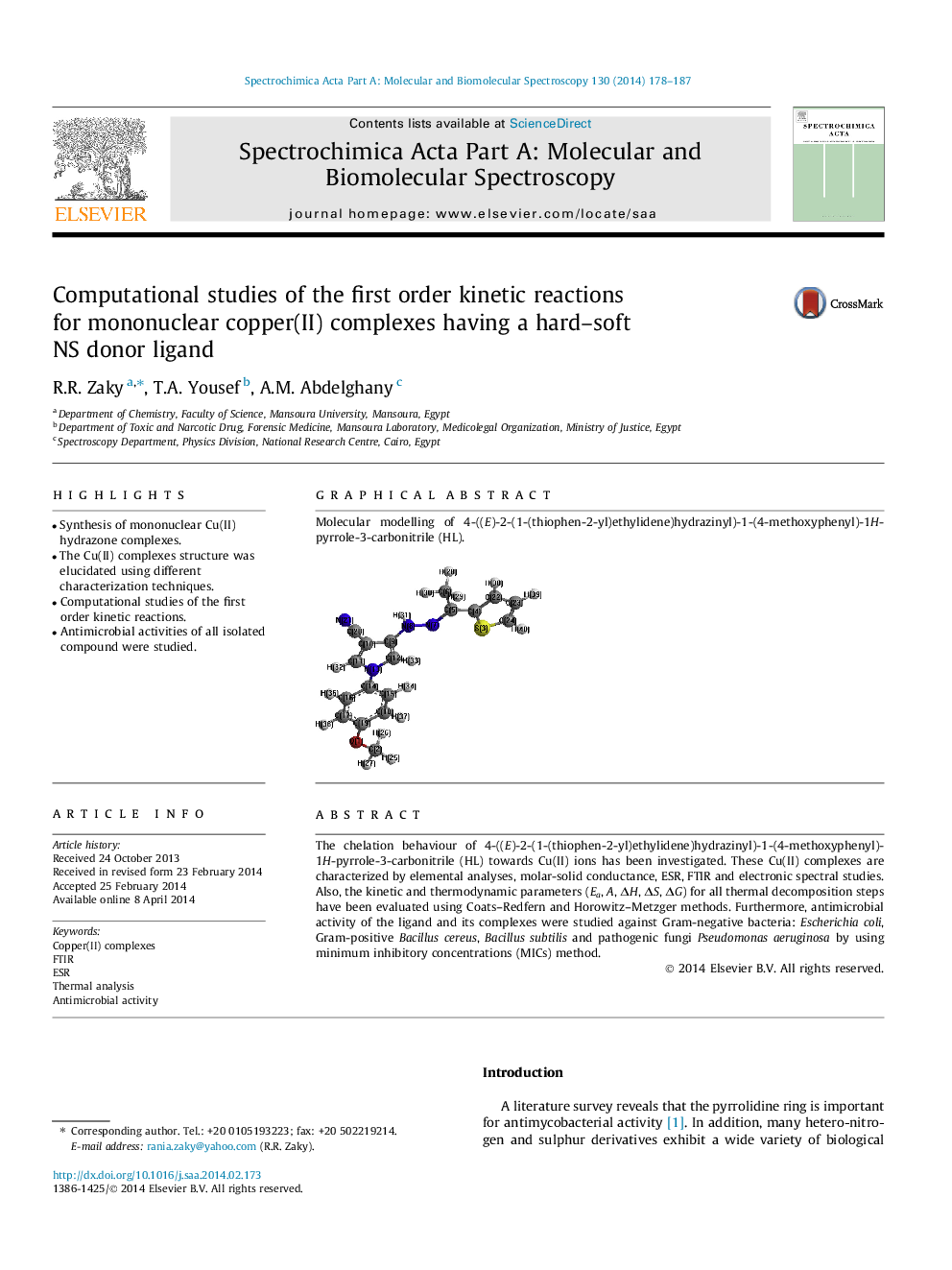
Molecular modelling of 4-((E)-2-(1-(thiophen-2-yl)ethylidene)hydrazinyl)-1-(4-methoxyphenyl)-1H-pyrrole-3-carbonitrile (HL).Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 130, 15 September 2014, Pages 178–187