| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232962 | 968800 | 2015 | 5 صفحه PDF | دانلود رایگان |
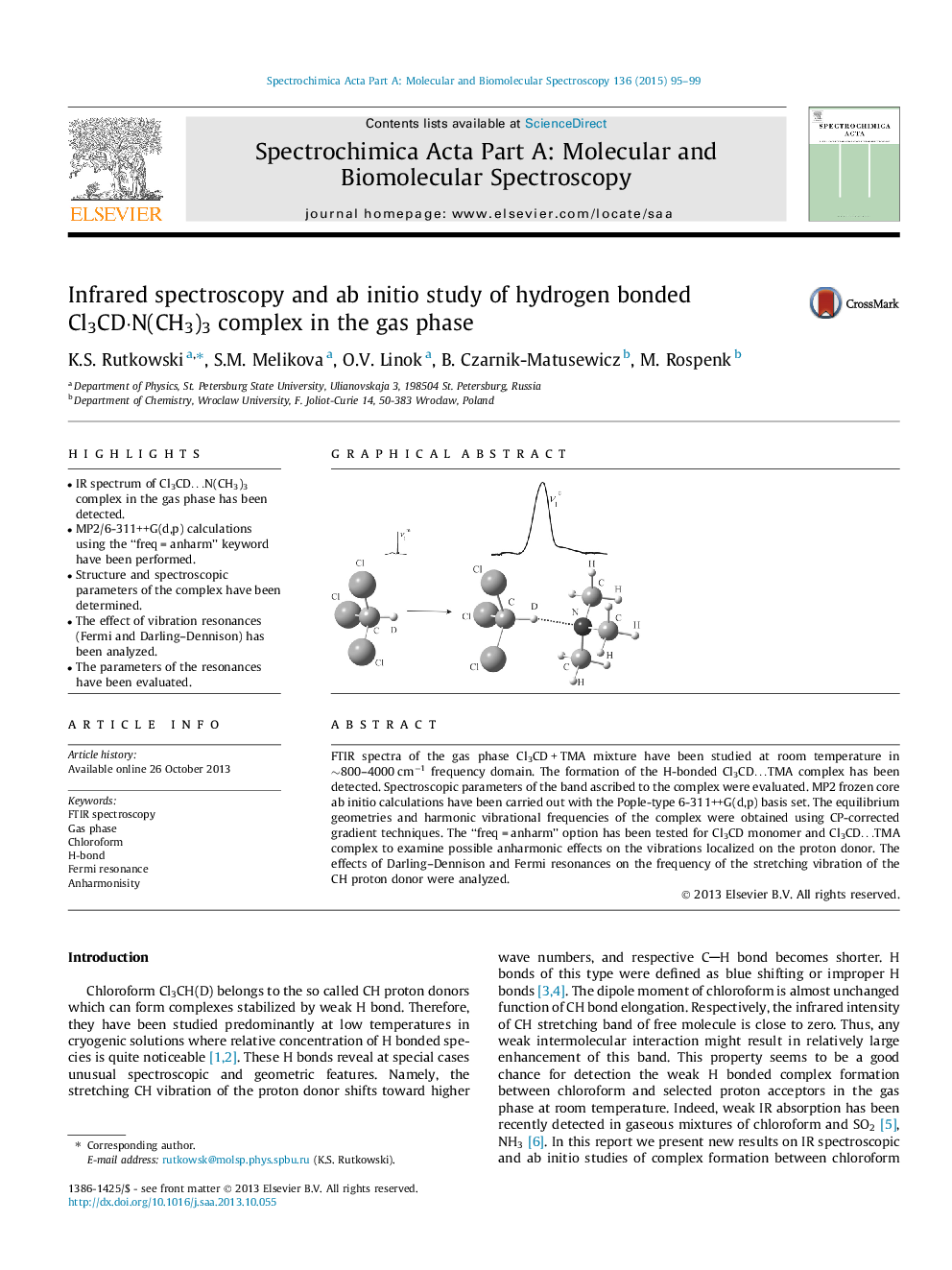
• IR spectrum of Cl3CD…N(CH3)3 complex in the gas phase has been detected.
• MP2/6-311++G(d,p) calculations using the “freq = anharm” keyword have been performed.
• Structure and spectroscopic parameters of the complex have been determined.
• The effect of vibration resonances (Fermi and Darling–Dennison) has been analyzed.
• The parameters of the resonances have been evaluated.
FTIR spectra of the gas phase Cl3CD + TMA mixture have been studied at room temperature in ∼800–4000 cm−1 frequency domain. The formation of the H-bonded Cl3CD…TMA complex has been detected. Spectroscopic parameters of the band ascribed to the complex were evaluated. MP2 frozen core ab initio calculations have been carried out with the Pople-type 6-311++G(d,p) basis set. The equilibrium geometries and harmonic vibrational frequencies of the complex were obtained using CP-corrected gradient techniques. The ‘‘freq = anharm’’ option has been tested for Cl3CD monomer and Cl3CD…TMA complex to examine possible anharmonic effects on the vibrations localized on the proton donor. The effects of Darling–Dennison and Fermi resonances on the frequency of the stretching vibration of the CH proton donor were analyzed.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part A, 5 February 2015, Pages 95–99