| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233239 | 1495233 | 2015 | 7 صفحه PDF | دانلود رایگان |
• β-Nickel hydroxide (β-Ni(OH)2) was synthesized using precipitation method.
• FT-IR and TG–DTA studies show that the β-Ni(OH)2 contains water molecules and anions.
• Electrochemical performance of β-Ni(OH)2 was investigated using CV and EIS.
• The proton diffusion coefficient (D) for β-Ni(OH)2 is found to be 1.44 × 10−12 cm2 s−1.
• EIS studies confirmed that the electrode reaction processes are diffusion controlled.
β-Nickel hydroxide (β-Ni(OH)2) was successfully synthesized using precipitation method. The structure and property of the β-Ni(OH)2 were characterized by X-ray diffraction (XRD), Fourier Transform infra-red (FT-IR), Raman spectra and thermal gravimetric–differential thermal analysis (TG–DTA). The results of the FTIR spectroscopy and TG–DTA studies indicate that the β-Ni(OH)2 contains water molecules and anions. The microstructural and composition studies have been performed using Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) analysis. A pasted-type electrode is prepared using β-Ni(OH)2 powder as the active material on a nickel sheet as a current collector. Cyclic voltammetry (CV) and Electrochemical impedance spectroscopy (EIS) studies were performed to evaluate the electrochemical performance of the β-Ni(OH)2 electrode in 6 M KOH electrolyte. CV curves showed a pair of strong redox peaks as a result of the Faradaic redox reactions of β-Ni(OH)2. The proton diffusion coefficient (D) for the present β-Ni(OH)2 electrode material is found to be 1.44 × 10−12 cm2 s−1. Further, electrochemical impedance studies confirmed that the β-Ni(OH)2 electrode reaction processes are diffusion controlled.
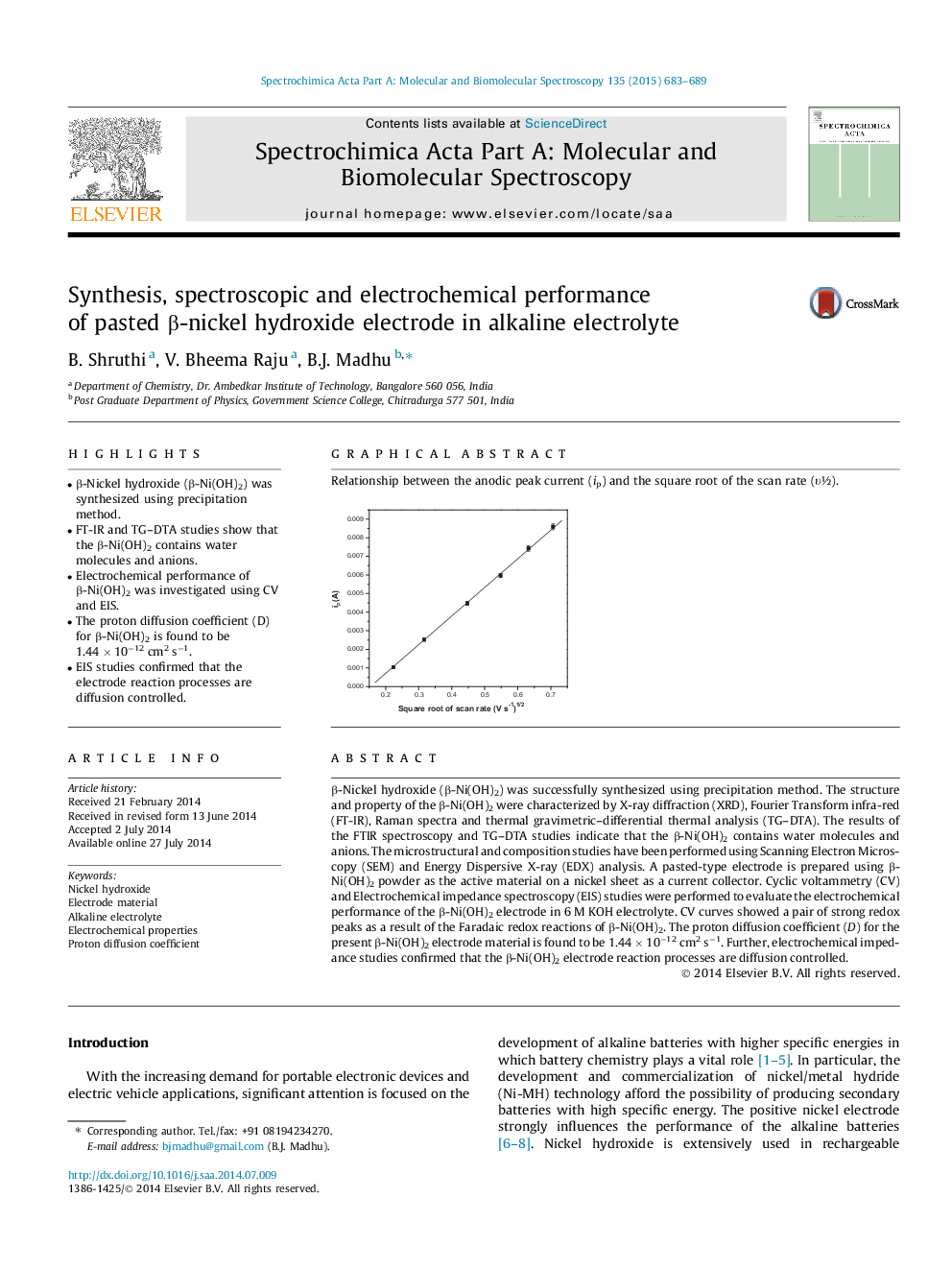
Relationship between the anodic peak current (ip) and the square root of the scan rate (υ½).Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 135, 25 January 2015, Pages 683–689