| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1234381 | 1495272 | 2012 | 12 صفحه PDF | دانلود رایگان |
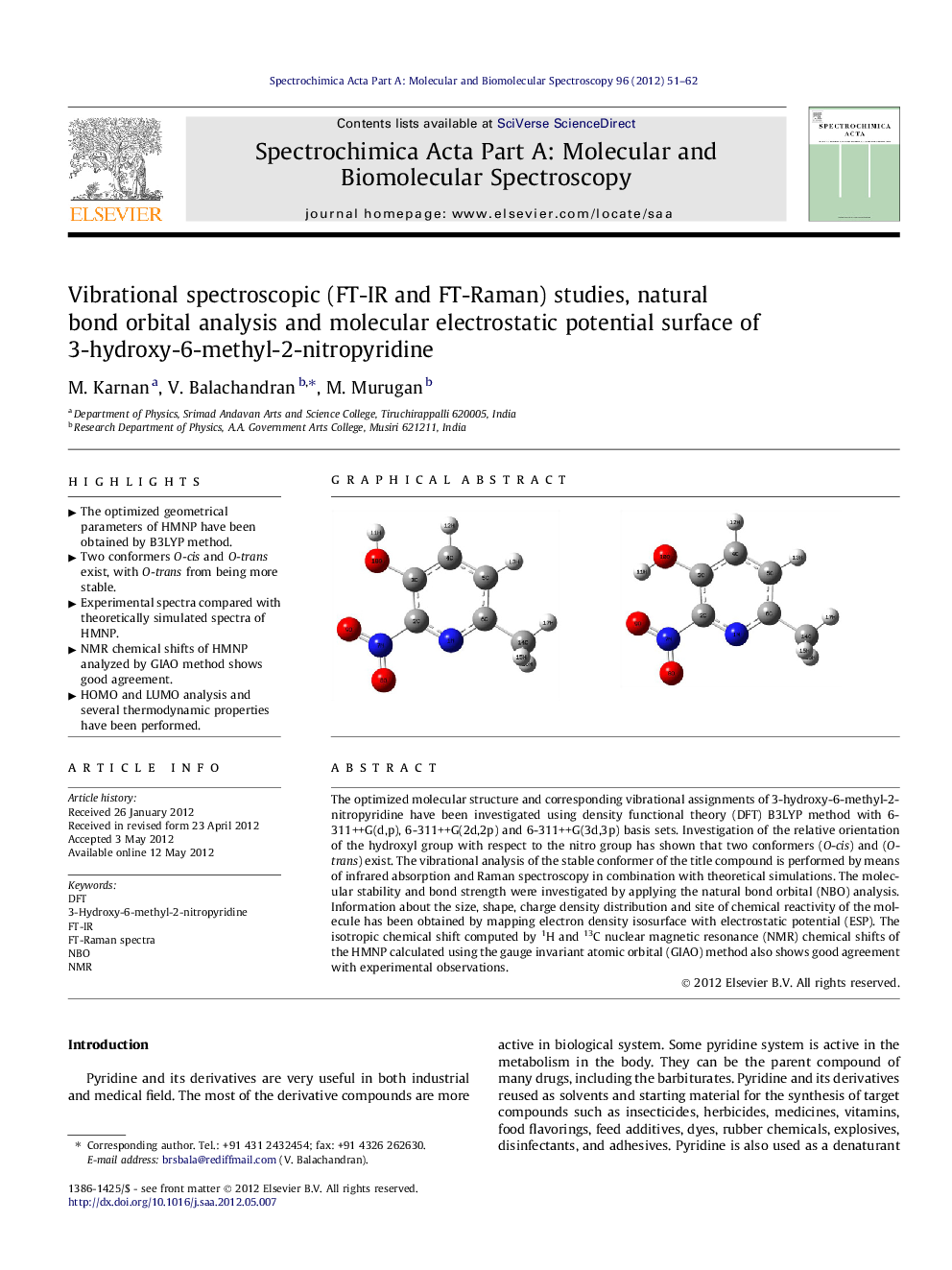
The optimized molecular structure and corresponding vibrational assignments of 3-hydroxy-6-methyl-2-nitropyridine have been investigated using density functional theory (DFT) B3LYP method with 6-311++G(d,p), 6-311++G(2d,2p) and 6-311++G(3d,3p) basis sets. Investigation of the relative orientation of the hydroxyl group with respect to the nitro group has shown that two conformers (O-cis) and (O-trans) exist. The vibrational analysis of the stable conformer of the title compound is performed by means of infrared absorption and Raman spectroscopy in combination with theoretical simulations. The molecular stability and bond strength were investigated by applying the natural bond orbital (NBO) analysis. Information about the size, shape, charge density distribution and site of chemical reactivity of the molecule has been obtained by mapping electron density isosurface with electrostatic potential (ESP). The isotropic chemical shift computed by 1H and 13C nuclear magnetic resonance (NMR) chemical shifts of the HMNP calculated using the gauge invariant atomic orbital (GIAO) method also shows good agreement with experimental observations.
Figure optionsDownload as PowerPoint slideHighlights
► The optimized geometrical parameters of HMNP have been obtained by B3LYP method.
► Two conformers O-cis and O-trans exist, with O-trans from being more stable.
► Experimental spectra compared with theoretically simulated spectra of HMNP.
► NMR chemical shifts of HMNP analyzed by GIAO method shows good agreement.
► HOMO and LUMO analysis and several thermodynamic properties have been performed.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 96, October 2012, Pages 51–62