| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1287703 | 1497995 | 2013 | 4 صفحه PDF | دانلود رایگان |
• The adsorption and diffusion properties of sulfur on the Ni/YSZ are studied.
• Adsorbed sulfur doesn't favor to be located at the stoichiometric Ni/YSZ interface.
• The adsorbed S− is oxidized to S2− and trapped at the oxygen vacancy.
• The trapped sulfur is very difficult to be removed by the fuel (e.g., H2).
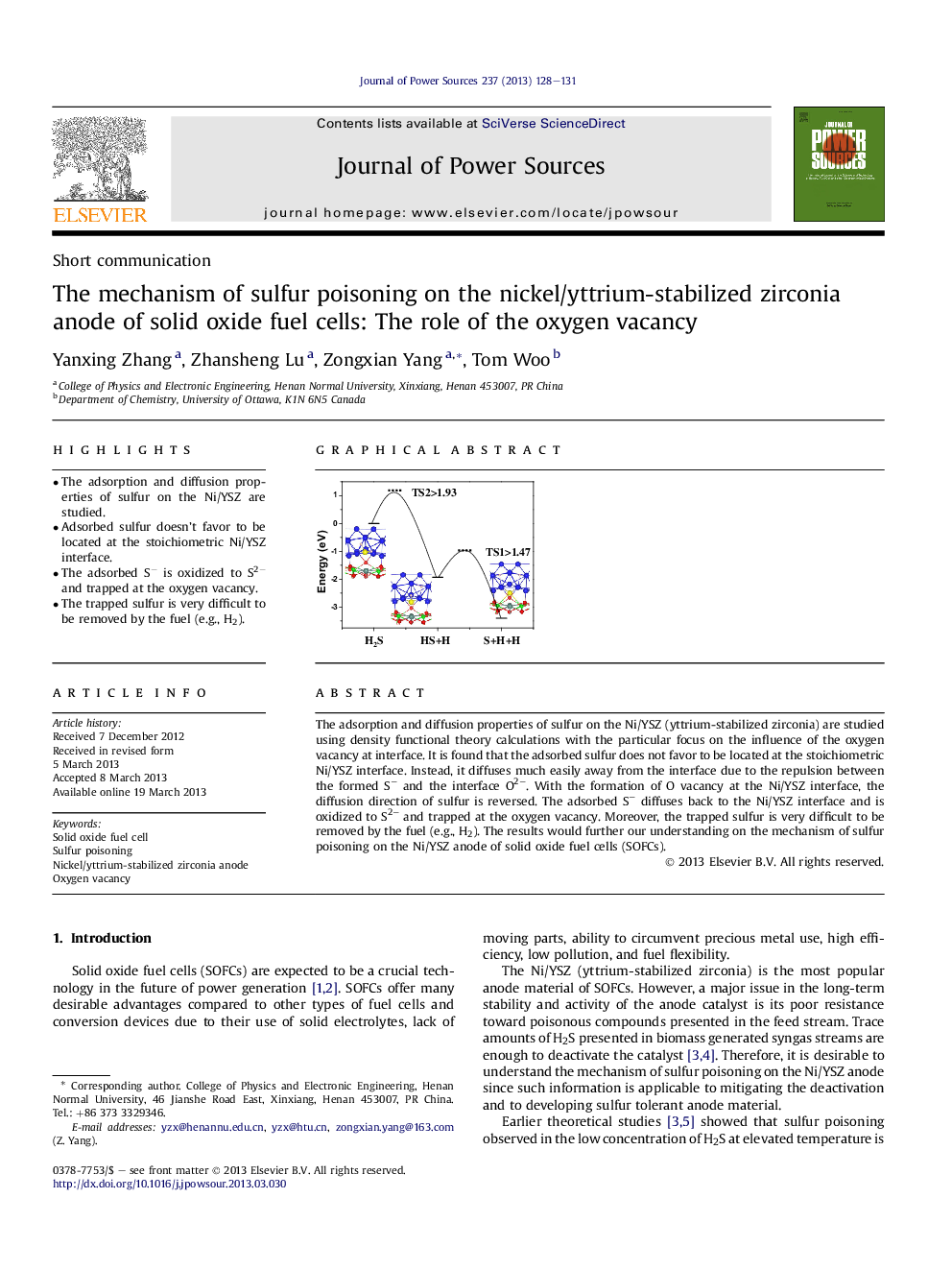
The adsorption and diffusion properties of sulfur on the Ni/YSZ (yttrium-stabilized zirconia) are studied using density functional theory calculations with the particular focus on the influence of the oxygen vacancy at interface. It is found that the adsorbed sulfur does not favor to be located at the stoichiometric Ni/YSZ interface. Instead, it diffuses much easily away from the interface due to the repulsion between the formed S− and the interface O2−. With the formation of O vacancy at the Ni/YSZ interface, the diffusion direction of sulfur is reversed. The adsorbed S− diffuses back to the Ni/YSZ interface and is oxidized to S2− and trapped at the oxygen vacancy. Moreover, the trapped sulfur is very difficult to be removed by the fuel (e.g., H2). The results would further our understanding on the mechanism of sulfur poisoning on the Ni/YSZ anode of solid oxide fuel cells (SOFCs).
With the formation of the O vacancy at the Ni/YSZ interface, the adsorbed S is oxidized to S2− and trapped at the oxygen vacancy. The trapped sulfur is very difficult to be removed by the fuel (e.g. H2). “…” represents the supposed barriers.Figure optionsDownload as PowerPoint slide
Journal: Journal of Power Sources - Volume 237, 1 September 2013, Pages 128–131